|
受注:045-509-1970 |
技術サポート:[email protected] 平日9:00〜18:00 1営業日以内にご連絡を差し上げます |
化学情報
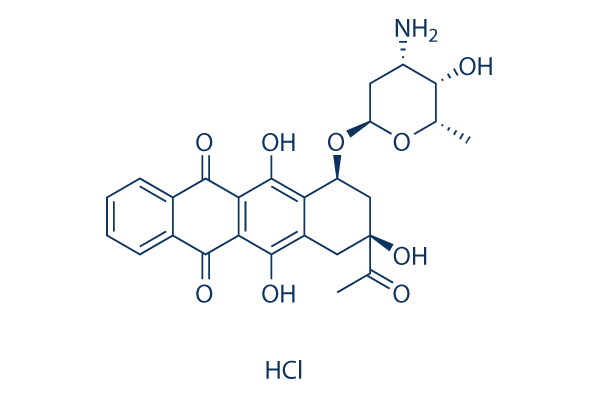
|
Synonyms | 4-demethoxydaunorubicin (NSC256439, 4-DMDR) HCl | Storage (From the date of receipt) |
3 years -20°C powder 1 years -80°C in solvent |
| 化学式 | C26H27NO9.HCl |
|||
| 分子量 | 533.95 | CAS No. | 57852-57-0 | |
| Solubility (25°C)* | 体外 | DMSO | 100 mg/mL (187.28 mM) | |
| Water | 10 mg/mL warmed with 50ºC water bath (18.72 mM) | |||
| Ethanol | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. |
||||
溶剤液(一定の濃度)を調合する
生物活性
| 製品説明 | Idarubicin HCl (4-demethoxydaunorubicin (NSC256439, 4-DMDR) HCl) is a hydrochloride salt form of Idarubicin which is an anthracycline antibiotic and a DNA topoisomerase II (topo II) inhibitor for MCF-7 cells with IC50 of 3.3 ng/mL in a cell-free assay. Idarubicin induces mTOR-dependent cytotoxic autophagy. |
|---|---|
| in vitro | Idarubicin has significant cytotoxic activity against multicellular spheroids, comparable to the antiproliferative effects on monolayer cells. [1] Idarubicin inhibits CYP450 2D6.[2] Idarubicin is about 57.5-fold and 25-fold more active than doxorubicin and epirubicin, respectively. Idarubicin is able to overcome P-glycoprotein-mediated multidrug resistance. [3] Idarubicin inhibits PMN superoxide radical formation. [4] Idarubicin could be coupled to the monoclonal antibodies (anti-Ly-2.1, anti-L3T4, or anti-Thy-1) with retention of protein solubility and antibody activity. [5] Idarubicin inhibits the proliferation of NALM-6 cells with an IC50 of 12 nM. [6] |
| in vivo | Reduction of Idarubicin is dependent upon ketone reductases, and proceeds more stereoselectively than that of most ketones giving rise to the (13S)-epimer almost exclusively. The high stereospecificity in Idarubicin reduction might result from chiral induction due to the presence of asymmetric centres near to the carbonyl group in Idarubicin. [7] |
| 特徴 | Idarubicin is a substrate for CYP450 2D6 and 2C9. |
プロトコル(参考用のみ)
| キナーゼアッセイ | CYP450 metabolism experiments | |
|---|---|---|
| Evaluation of Idarubicin metabolism by the CYP450 isoenzymes 3A4, 2D6, 2C8, 2C9, and 1A2 is completed using isolated human CYP450 proteins for each isoform. The high throughput P450 inhibition testing method is utilized for these evaluations. The metabolism experiments are designed to investigate the following properties of each drug: (1) if Idarubicin is a substrate of the CYP450 3A4, 2C8, 2C9, 1A2 or 2D6 isoenzymes; (2) if metabolism is affected by known inhibitors of each isoenzyme; (3) if Idarubicin is inhibitors of CYP450 isoenzymes; and (4) if caspofungin or itraconazole inhibit the CYP450 metabolism of Idarubicin. Dibenzylfluorescein (DBF) (CYP3A4, CYP2C8, CYP2C9), 3-cyano-7-ethoxycoumarin (Cyp1A2), and 7-methoxy-4-(aminomethyl)-coumarin (MAMC) (CYP2D6) are the known substrates utilized as controls to confirm the respective isoenzyme activity and evaluate the effects of Idarubicin on the isoenzyme activity. In addition, ketoconazole, quercetin, suflaphenazole, furafylline, and quinidine are utilized as control CYP450 inhibitors for 3A4, 2C8, 2C9, 1A2 or 2D6 isoenzymes, respectively. The substrate, inhibitor plus Idarubicin as indicated are added to each protein sample are incubated for 20 minutes- 60 minutes, as recommend by manufacturer, at 37oC. Reactions are stopped with an organic solvent solution and then samples are analyzed by fluorescence plate reader as appropriate. For each experiment, control samples with a known amount of substrate and synthesized metabolite, in the absence of the isoenzyme, are prepared for qualitative comparisons. All experiments are performed in triplicate. | ||
| 細胞アッセイ | 細胞株 | NALM-6 cells |
| 濃度 | 0.1 nM-10 μM | |
| 反応時間 | 24 hours | |
| 実験の流れ | The anti-proliferative activity of the Idarubicin in the conjugate is compared to that of free drug by measuring the inhibition of [3H]thymidine uptake. Briefly, NALM-6 cells (1.5 × 106/mL) are added to a flat-bottomed microtitre plate (100 μL/well) and incubated for 1 hours at 37ºC. Free Idarubicin and Idarubicin-mAb conjugates are sterilised by filtration and diluted in sterile PBS; various concentrations are added to the wells (100 μL/well) in duplicate and the plates are incubated at 37ºC, 7% CO2 for 24 hours. Following incubation, 50 μL medium containing 1 μCi [3H]thymidine is added to each well and the plates are incubated for a further 4 hours. Cells are harvested onto glass-fibre filter-paper, dried and counted in a scintillation counter. Specificity studies are performed using the same technique where the ability of Idarubicin-anti-CD19 conjugates to kill CD19 + cells is compared to the cytotoxicity of irrelevant Idarubicin-JGT conjugates. NALM-6 cells (1.5× 106/mL, 300 μL tube) are incubated for 30 rain on ice with various concentrations of Idarubicin-anti-CD 19 or Idarubicin-JGT conjugates. Following three washes in ice-cold RPMI-1640 medium (4 mL/wash), the cells are resuspended in fresh medium and transferred to 96-well plates (100 μL/well). Each tube is set up in duplicate and two wells are plated out per tube (a total of 4 wells per drug concentration). Cells are pulsed with [3H]thymidine 24 hours later and harvested. |
|
| 動物実験 | 動物モデル | Rat, rabbit, mouse, dog |
| 投薬量 | 2 mg/kg, 0 mg/kg -75 mg/kg, 3 mg/kg and 0 mg/kg -75 mg/kg | |
| 投与方法 | Administered via i.v. | |
参考
カスタマーフィードバック
-
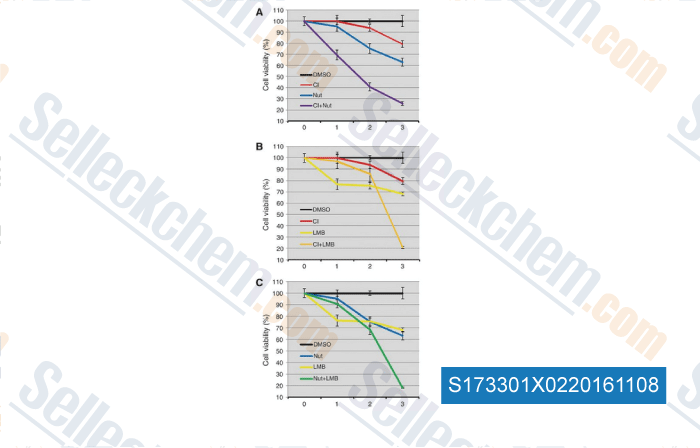
, , Clin Cancer Res, 2016, 22(3):746-56.

-
Data from [Data independently produced by , , Leukemia, 2018, 32(2):303-312]
-
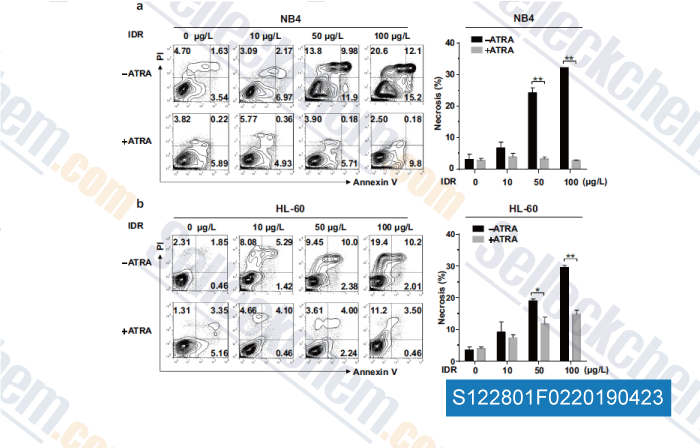
Data from [Data independently produced by , , Mol Cell Biochem, 2018, doi:10.1007/s11010-018-3402-0]
Selleckの高級品が、幾つかの出版された研究調査結果(以下を含む)で使われた:
| Histone H3 lysine 27 crotonylation mediates gene transcriptional repression in chromatin [ Mol Cell, 2023, 83(13):2206-2221.e11] | PubMed: 37311463 |
| Spermatogenesis associated serine rich 2 like plays a prognostic factor and therapeutic target in acute myeloid leukemia by regulating the JAK2/STAT3/STAT5 axis [ J Transl Med, 2023, 21(1):115] | PubMed: 36774517 |
| Relevance of the organic anion transporting polypeptide 1B3 (OATP1B3) in the personalized pharmacological treatment of hepatocellular carcinoma [ Biochem Pharmacol, 2023, 214:115681] | PubMed: 37429423 |
| p53 controls choice between apoptotic and non-apoptotic death following DNA damage [ bioRxiv, 2023, 2023.01.17.524444] | PubMed: 36712034 |
| Acquired mutations in BAX confer resistance to BH3-mimetic therapy in Acute Myeloid Leukemia [ Blood, 2022, blood.2022016090] | PubMed: 36219880 |
| Establishment and large-scale validation of a three-dimensional tumor model on an array chip for anticancer drug evaluation [ Front Pharmacol, 2022, 13:1032975] | PubMed: 36313330 |
| Establishment and Characterization of NCC-PMP1-C1: A Novel Patient-Derived Cell Line of Metastatic Pseudomyxoma Peritonei [ J Pers Med, 2022, 12(2)258] | PubMed: 35207746 |
| Topoisomerase II as a Novel Antiviral Target against Panarenaviral Diseases [ Viruses, 2022, 15(1)105] | PubMed: 36680145 |
| Establishment and characterization of NCC-UPS4-C1: a novel cell line of undifferentiated pleomorphic sarcoma from a patient with Li-Fraumeni syndrome [ Hum Cell, 2022, 10.1007/s13577-022-00671-y] | PubMed: 35118583 |
| Cancer-Targeted Controlled Delivery of Chemotherapeutic Anthracycline Derivatives Using Apoferritin Nanocage Carriers [ Int J Mol Sci, 2021, 22(3)1362] | PubMed: 33572999 |
長期の保管のために-20°Cの下で製品を保ってください。
人間や獣医の診断であるか治療的な使用のためにでない。
各々の製品のための特定の保管と取扱い情報は、製品データシートの上で示されます。大部分のSelleck製品は、推薦された状況の下で安定です。製品は、推薦された保管温度と異なる温度で、時々出荷されます。長期の保管のために必要とされてそれと異なる温度で、多くの製品は、短期もので安定です。品質を維持するが、夜通しの積荷のために最も経済的な貯蔵状況を用いてあなたの送料を保存する状況の下に、製品が出荷されることを、我々は確実とします。製品の受領と同時に、製品データシートの上で貯蔵推薦に従ってください。
