|
受注:045-509-1970 |
技術サポート:[email protected] 平日9:00〜18:00 1営業日以内にご連絡を差し上げます |
化学情報
|
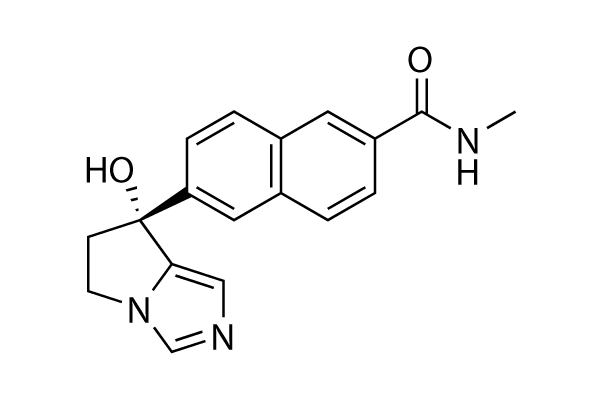
Synonyms | TAK-700 | Storage (From the date of receipt) |
3 years -20°C powder 1 years -80°C in solvent |
| 化学式 | C18H17N3O2 |
|||
| 分子量 | 307.35 | CAS No. | 426219-18-3 | |
| Solubility (25°C)* | 体外 | DMSO | 61 mg/mL (198.47 mM) | |
| Ethanol | 8 mg/mL (26.02 mM) | |||
| Water | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. |
||||
溶剤液(一定の濃度)を調合する
生物活性
| 製品説明 | Orteronel(TAK-700) is a potent and highly selective human 17,20-lyase inhibitor with IC50 of 38 nM, exhibits >1000-fold selectivity over other CYPs (e.g. 11-hydroxylase and CYP3A4). TAK-700 (Orteronel) is an androgen biosynthesis inhibitor. Phase 3. |
|---|---|
| in vitro | In vitro, TAK-700 shows the potent inhibitory activity against rat and human steroid 17,20-lyase with IC50 of 54 nM and 38 nM, respectively. While other CYP isoforms including 11-hydroxylase and CYP3A4 are not significantly affected by TAK-700. [1] In microsomes expressing human CYP isoforms, TAK-700 exhibit greater inhibitory effects on 17,20-lyase with IC50 of 19 nM compared to the other CYP isoforms. [1] TAK-700 shows the inhibitory activity against monkey 17,20-lyase and 17-hydroxylase with IC50 of 27 nM and 38 nM, respectively. [2] In monkey adrenal cells, TAK-700 inhibits the ACTH stimulated production of DHEA and androstenedione with IC50 of 110 nM and 130 nM, respectively. Moreover, TAK-700 also potently inhibits DHEA production in human adrenocortical tumor line H295R cells with IC50 of 37 nM. [2] |
| in vivo | In cynomolgus monkeys, oral treatment of TAK-700 at a dose of 1 mg/kg markedly reduces serum testosterone and dehydroepiandrosterone (DHEA) levels. [1] Oral treatment of TAK-700 at a dose of 1 mg/kg results in favorable pharmacokinetic parameters with Tmax, Cmax, t1/2 and AUC0-24 hours of 1.7 hours, 0.147 μg/mL, 3.8 hours and 0.727 μg h/mL, respectively. [2] |
プロトコル(参考用のみ)
| キナーゼアッセイ | Inhibitory activity on steroid 17,20-lyase in vitro | |
|---|---|---|
| Testes excised from 13-week-old male SD rats are homogenized, and testicular microsomes are prepared by centrifugation. The reaction mixture contained 75 mM phosphate buffer (pH 7.4), 7 μg of the microsome protein, 10 nM [1,2-3H]-17α-hydroxyprogesterone (NEN), 5 mM NADPH (Oriental Yeast), and 1 nM-1000 nM TAK-700 in a total volume of 20 μL at room temperature. The concentration of reagents is expressed as the final concentration in the reaction mixture. TAK-700 is serially diluted with dimethylformamide, and then diluted fivefold with distilled water before 5 μL of the diluted solution is added to the reaction mixture. After incubating for 15 minutes at 37 °C the reaction is terminated by the addition of 40 μL of ethyl acetate, then vortexed for 30 seconds and briefly centrifuged. Thirty microliters of the organic phases are applied to silica gel TLC plates. The substrate and the products, androstenedione and testosterone, are separated using the toluene-acetone (7:2) solvent system. Detection of the spots and measurement of the radioactivity as PSL are performed with a BAS2000 Bio-image analyzer (FUJIX). The concentration of TAK-700 needed to reduce the concentration of the products by 50% (the concentration of the control group in which no TAK-700 is added is taken as 100%) is calculated. Inhibitory activity on human enzymes is determined as described above. The reaction mixture contained 75 mM phosphate buffer (pH 7.4), 1 mM magnesium chloride, 0.5 pmol of recombinant P450c17, 0.5 pmol of recombinant cytochrome b5, 20.8 ng of recombinant NADPH-cytochrome P450 reductase, 10 nM [1,2-3H]-17α-hydroxypregnenolone, 5 mM NADPH (Oriental Yeast), and 1 nM-1000 nM test compound in a total volume of 20 μL. The concentration of reagents is expressed as the final concentration in the reaction mixture. TAK-700 is serially diluted with dimethylformamide, and then diluted fivefold with distilled water before 5 μL of the diluted solution is added to the reaction mixture. After incubating for 15 minutes at 37 °C the reaction is terminated by the addition of 40 μL of ethyl acetate, then vortexed for 30 seconds and briefly centrifuged. Thirty microliters of the organic phases are applied to silica gel TLC plates. The substrate and the product DHEA are separated using the cyclohexane-ethyl acetate (3:2) solvent system. | ||
| 動物実験 | 動物モデル | Adult male cynomolgus monkeys. |
| 投薬量 | ≤1 mg/kg | |
| 投与方法 | Administered via p.o. | |
参考
|
Selleckの高級品が、幾つかの出版された研究調査結果(以下を含む)で使われた:
| Virtual screening and biological evaluation to identify pharmaceuticals potentially causing hypertension and hypokalemia by inhibiting steroid 11β-hydroxylase [ Toxicol Appl Pharmacol, 2023, 475:116638] | PubMed: 37499767 |
| Structural and Functional Evaluation of Clinically Relevant Inhibitors of Steroidogenic Cytochrome P450 17A1. [ Drug Metab Dispos, 2017, 45(6):635-645] | PubMed: 28373265 |
| Specificity of anti-prostate cancer CYP17A1 inhibitors on androgen biosynthesis [Udhane SS, et al. Biochem Biophys Res Commun, 2016, 477(4):1005-10] | PubMed: 27395338 |
長期の保管のために-20°Cの下で製品を保ってください。
人間や獣医の診断であるか治療的な使用のためにでない。
各々の製品のための特定の保管と取扱い情報は、製品データシートの上で示されます。大部分のSelleck製品は、推薦された状況の下で安定です。製品は、推薦された保管温度と異なる温度で、時々出荷されます。長期の保管のために必要とされてそれと異なる温度で、多くの製品は、短期もので安定です。品質を維持するが、夜通しの積荷のために最も経済的な貯蔵状況を用いてあなたの送料を保存する状況の下に、製品が出荷されることを、我々は確実とします。製品の受領と同時に、製品データシートの上で貯蔵推薦に従ってください。
