|
受注:045-509-1970 |
技術サポート:[email protected] 平日9:00〜18:00 1営業日以内にご連絡を差し上げます |
化学情報
|
Synonyms | CM 9155 | Storage (From the date of receipt) |
3 years -20°C powder 1 years -80°C in solvent |
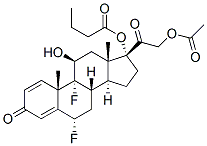
| 化学式 | C27H34F2O7 |
|||
| 分子量 | 508.55 | CAS No. | 23674-86-4 | |
| Solubility (25°C)* | 体外 | DMSO | 102 mg/mL (200.57 mM) | |
| Ethanol | 25 mg/mL (49.15 mM) | |||
| Water | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. |
||||
溶剤液(一定の濃度)を調合する
生物活性
| 製品説明 | Difluprednate (difluoroprednisolone butyrate acetate, DFBA, CM 9155) is a synthetic difluorinated prednisolone derivative, it is originally developed for dermatologic applications. |
|---|---|
| in vitro | The difluprednate emulsion formulation begin by suspending DFBA in a variety of oils (castor, cottonseed, medium-chain fatty acid triglyceride, oleic, olive, peanut, and soybean). [1] Difluprednate ophthalmic emulsion's active metabolite, 6α,9-difluoroprednisolone 17-butyrate (DFB), has the lowest Ki value (611 nM) for the glucocorticoid receptor and is significantly more active than prednisolone, which has a Ki value of 3.4 nM. [2] |
| in vivo | Difluprednate emulsion is rapidly deacetylated in the aqueous humor to difluoroprednisolone butyrate (DFB) in rats and rabbits, the drug’s active metabolite, which has a similar corticosteroid activity profile. Radio-labeled difluprednate is instilled in the right eyes of pigmented rabbits in a separate study examining DFBA excretion. Difluprednate (0.05% emulsion) results in higher aqueous humor concentrations than the suspension form in the eyes of rabbits. Difluprednate concentrations ≥0.01% shows a statistically significant inhibition of inflammation compared to saline in a rabbit model, with the anti-inflammatory response proceeding in a dose-dependent manner. Difluprednate suppresses uveitis in all three concentration (0.002%, 0.01%, and 0.05%) in a dose-dependent manner in rats, and difluprednate 0.05% shows statistically superior anti-inflammatory activity compared to Betamethasone (0.1%). [1] |
プロトコル(参考用のみ)
参考
|
Selleckの高級品が、幾つかの出版された研究調査結果(以下を含む)で使われた:
| Development and validation of a high-performance liquid chromatography method for the quantification of talazoparib in rat plasma: Application to plasma protein binding studies [ Biomed Chromatogr, 2018, 32(2)] | PubMed: 28677821 |
長期の保管のために-20°Cの下で製品を保ってください。
人間や獣医の診断であるか治療的な使用のためにでない。
各々の製品のための特定の保管と取扱い情報は、製品データシートの上で示されます。大部分のSelleck製品は、推薦された状況の下で安定です。製品は、推薦された保管温度と異なる温度で、時々出荷されます。長期の保管のために必要とされてそれと異なる温度で、多くの製品は、短期もので安定です。品質を維持するが、夜通しの積荷のために最も経済的な貯蔵状況を用いてあなたの送料を保存する状況の下に、製品が出荷されることを、我々は確実とします。製品の受領と同時に、製品データシートの上で貯蔵推薦に従ってください。
