|
受注:045-509-1970 |
技術サポート:[email protected] 平日9:00〜18:00 1営業日以内にご連絡を差し上げます |
化学情報
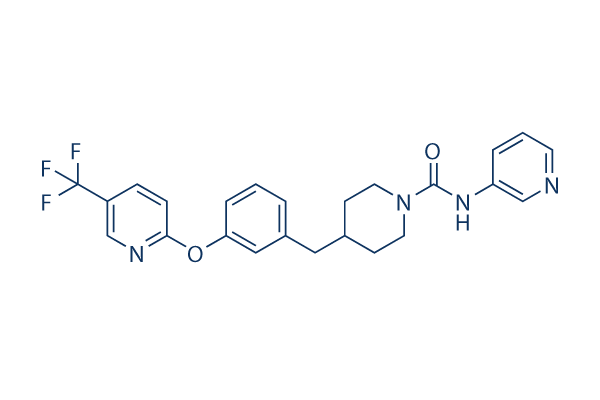
|
Synonyms | N/A | Storage (From the date of receipt) |
3 years -20°C powder 1 years -80°C in solvent |
| 化学式 | C24H23F3N4O2 |
|||
| 分子量 | 456.46 | CAS No. | 1196109-52-0 | |
| Solubility (25°C)* | 体外 | DMSO | 91 mg/mL (199.36 mM) | |
| Ethanol | 91 mg/mL (199.36 mM) | |||
| Water | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. |
||||
溶剤液(一定の濃度)を調合する
生物活性
| 製品説明 | PF-3845 is a potent, selective and irreversible FAAH inhibitor with Ki of 230 nM, showing negligible activity against FAAH2. |
|---|---|
| in vitro | PF-3845 selectively inhibits FAAH by carbamylating FAAH's serine nucleophile. [1] |
| in vivo | PF-3845 treated mice (10 mg/kg, i.p.) shows rapid and complete inactivation of FAAH in the brain, as judged by competitive activity-based protein profiling (ABPP) with the serine hydrolase-directed probe fluorophosphonate (FP)-rhodamine. PF-3845 shows a long duration of action up to 24 hour. PF-3845-treated mice also shows dramatic (>10-fold) elevation in brain levels of AEA and other NAEs (N-pamitoyl ethanolamine [PEA] and N-oleoyl ethanolamine [OEA]). FAAH is AEA-degrading enzyme fatty acid amide hydrolase. PF-3845 (1–30 mg/kg, oral administration [p.o.]) causes a dose dependent inhibition of mechanical allodynia with a minimum effective dose (MED) of 3 mg/kg (rats are analyzed at 4 hour post dosing with PF-3845). At higher doses (10 and 30 mg/kg), PF-3845 inhibits pain responses to an equivalent, if not greater, degree than the nonsteroidal anti-inflammatory drug naproxen (10mg/kg, p.o.). [1] PF-3845 (10 mg/kg, i.p.) significantly reverses LPS-induced tactile allodynia, but doesn't modify paw withdrawal thresholds in the saline-injected paw. [2] |
プロトコル(参考用のみ)
| キナーゼアッセイ | FAAH Assay | |
|---|---|---|
| The GDH-coupled FAAH assay is performed for determination of potencies (Kinact/ Ki values) of irreversible inhibitors. For the development of structure-activity relationship, the FAAH assay is performed in 384-well microplates with a final volume of 50μL. In the 384-well format assay, the overall potency, Kinact/ Ki values, are generally calculated from the slope, Kinact/[ Ki (1 + [S]/ Km)], which is obtained from the kobs versus [I] linear lines under the conditions of [I] << Ki as described (from the kobs values fitted to equation 3 in PNAS 2008). In order to obtain Kinact and Ki values separately for PF-3845 and URB597, the FAAH assay is performed in the 96-well format with a final volume of 200μL. Due to its more frequent reading capabilities (every 10 and 30 seconds for the 96- and 384-well formats, respectively), the 96- well format allows a measurement of Kobs values at higher concentrations of inhibitors. The Kinact and Ki values are obtained by fitting the kobs versus [I] curves to the equation, Kobs = Kinact [I]/{[I] + Ki (1 + [S]/ Km}. FAAH inactivation rates in the absence of inhibitors are subtracted from all kobs values obtained in the presence of inhibitors. The ratio Kinact/ Ki calculated from the individual Kinact and Ki values obtained separately agrees well with the Kinact/ Ki value obtained from the slope described above. | ||
| 動物実験 | 動物モデル | Male C57BL/6 mice |
| 投薬量 | 10 mg/kg or 1-30 mg/kg | |
| 投与方法 | Administered via i.p. 1 hour before sacrificed by CO2 or oral administration. | |
参考
|
Selleckの高級品が、幾つかの出版された研究調査結果(以下を含む)で使われた:
| Cooperative Enzymatic Control of N-acyl Amino Acids by PM20D1 and FAAH [ Elife, 2020, 9;9:e55211] | PubMed: 32271712 |
| Modulation of Pain Sensitivity by Chronic Consumption of Highly Palatable Food Followed by Abstinence: Emerging Role of Fatty Acid Amide Hydrolase. [ Front Pharmacol, 2020, 11:266] | PubMed: 32231568 |
| The fatty-acid amide hydrolase inhibitor URB597 inhibits MICA/B shedding [ Sci Rep, 2020, 10(1):15556] | PubMed: 32968163 |
| Bioluminescent cell-based NAD(P)/NAD(P)H assays for rapid dinucleotide measurement and inhibitor screening [ Assay Drug Dev Technol, 2014, 12(9-10):514-26] | PubMed: 25506801 |
長期の保管のために-20°Cの下で製品を保ってください。
人間や獣医の診断であるか治療的な使用のためにでない。
各々の製品のための特定の保管と取扱い情報は、製品データシートの上で示されます。大部分のSelleck製品は、推薦された状況の下で安定です。製品は、推薦された保管温度と異なる温度で、時々出荷されます。長期の保管のために必要とされてそれと異なる温度で、多くの製品は、短期もので安定です。品質を維持するが、夜通しの積荷のために最も経済的な貯蔵状況を用いてあなたの送料を保存する状況の下に、製品が出荷されることを、我々は確実とします。製品の受領と同時に、製品データシートの上で貯蔵推薦に従ってください。
