|
受注:045-509-1970 |
技術サポート:[email protected] 平日9:00〜18:00 1営業日以内にご連絡を差し上げます |
化学情報
|
Synonyms | A77 1726, HMR-1726 | Storage (From the date of receipt) |
3 years -20°C powder 1 years -80°C in solvent |
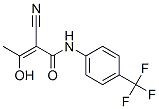
| 化学式 | C12H9F3N2O2 |
|||
| 分子量 | 270.21 | CAS No. | 163451-81-8 | |
| Solubility (25°C)* | 体外 | DMSO | 54 mg/mL (199.84 mM) | |
| Water | Insoluble | |||
| Ethanol | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. |
||||
溶剤液(一定の濃度)を調合する
生物活性
| 製品説明 | Teriflunomide (A77 1726, HMR-1726) is the active metabolite of leflunomide, inhibiting pyrimidine de novo synthesis by blocking the enzyme dihydroorotate dehydrogenase, used as an immunomodulatory agent. |
|---|---|
| in vitro | Teriflunomide primarily acts as an inhibitor of dihydroorotate dehydrogenase (DHODH), a key mitochondrial enzyme involved in the de novo synthesis of pyrimidines in rapidly proliferating cells. By reducing the activity of high-avidity proliferating T lymphocytes and B lymphocytes, teriflunomide likely attenuates the inflammatory response to autoantigens in MS. Thus, teriflunomide can be considered a cytostatic rather than a cytotoxic drug to leukocytes. [1] |
| in vivo | Teriflunomide has demonstrated beneficial effects in two independent animal models of demyelinating disease. In the dark agouti rat model of experimental autoimmune encephalitis (EAE), teriflunomide administration results in clinical, histopathological, and electrophysiological evidence of efficacy both as a prophylactic and therapeutic agent. Similarly, in the female Lewis rat model of EAE, teriflunomide administration results in beneficial prophylactic and therapeutic clinical effects, with a delay in disease onset and symptom severity. [1] |
プロトコル(参考用のみ)
カスタマーフィードバック
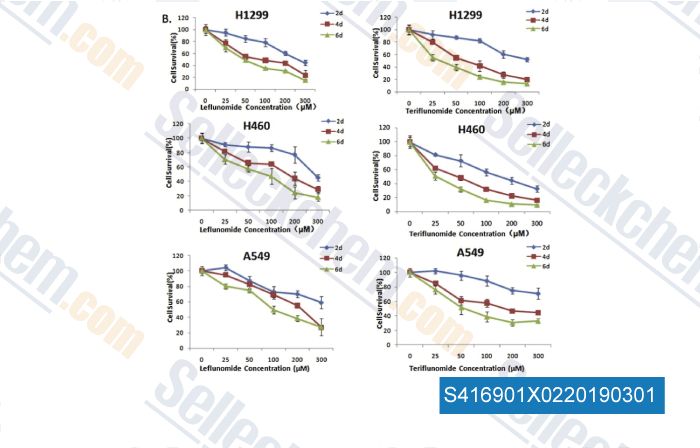
-
Data from [Data independently produced by , , Toxicol Lett, 2018, 282:154-165]
Selleckの高級品が、幾つかの出版された研究調査結果(以下を含む)で使われた:
| Selective vulnerability of human-induced pluripotent stem cells to dihydroorotate dehydrogenase inhibition during mesenchymal stem/stromal cell purification [ Front Cell Dev Biol, 2023, 11:1089945] | PubMed: 36814599 |
| Proof-of-principle studies on a strategy to enhance nucleotide imbalance specifically in cancer cells [ Cell Death Discov, 2022, 8(1):464] | PubMed: 36424385 |
| Inhibitors of dihydroorotate dehydrogenase cooperate with molnupiravir and N4-hydroxycytidine to suppress SARS-CoV-2 replication [ iScience, 2022, 25(5):104293] | PubMed: 35492218 |
| Inhibition of de novo pyrimidine synthesis augments Gemcitabine induced growth inhibition in an immunocompetent model of pancreatic cancer [ Int J Biol Sci, 2021, 17(9):2240-2251] | PubMed: 34239352 |
| The Mitochondria-Independent Cytotoxic Effect of Leflunomide on RPMI-8226 Multiple Myeloma Cell Line [ Molecules, 2021, 26(18)5653] | PubMed: 34577124 |
| Inhibition of canine distemper virus replication by blocking pyrimidine nucleotide synthesis with A77 1726, the active metabolite of the anti-inflammatory drug leflunomide [ J Gen Virol, 2021, 102(3)] | PubMed: 33416466 |
| Application of a newly-developed cynomolgus macaque BiTE-mediated cytotoxic T-lymphocyte activity assay to various immunomodulatory agents in vitro [ J Immunotoxicol, 2021, 18(1):154-162] | PubMed: 34714999 |
| N4-hydroxycytidine and inhibitors of dihydroorotate dehydrogenase synergistically suppress SARS-CoV-2 replication [ bioRxiv, 2021, 10.1101/2021.06.28.450163] | PubMed: N/A |
| N4-hydroxycytidine and inhibitors of dihydroorotate dehydrogenase synergistically suppress SARS-CoV-2 replication [ bioRxiv, 2021, 10.1101/2021.06.28.450163] | PubMed: None |
| Actionable Cytopathogenic Host Responses of Human Alveolar Type 2 Cells to SARS-CoV-2 [ Mol Cell, 2020, 80(6):1104-1122.e9] | PubMed: 33259812 |
長期の保管のために-20°Cの下で製品を保ってください。
人間や獣医の診断であるか治療的な使用のためにでない。
各々の製品のための特定の保管と取扱い情報は、製品データシートの上で示されます。大部分のSelleck製品は、推薦された状況の下で安定です。製品は、推薦された保管温度と異なる温度で、時々出荷されます。長期の保管のために必要とされてそれと異なる温度で、多くの製品は、短期もので安定です。品質を維持するが、夜通しの積荷のために最も経済的な貯蔵状況を用いてあなたの送料を保存する状況の下に、製品が出荷されることを、我々は確実とします。製品の受領と同時に、製品データシートの上で貯蔵推薦に従ってください。
