|
受注:045-509-1970 |
技術サポート:[email protected] 平日9:00〜18:00 1営業日以内にご連絡を差し上げます |
化学情報
|
Synonyms | N/A | Storage (From the date of receipt) |
3 years -20°C powder 1 years -80°C in solvent |
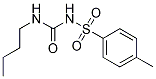
| 化学式 | C12H18N2O3S |
|||
| 分子量 | 270.35 | CAS No. | 64-77-7 | |
| Solubility (25°C)* | 体外 | DMSO | 54 mg/mL (199.74 mM) | |
| Ethanol | 54 mg/mL warmed with 50ºC water bath (199.74 mM) | |||
| Water | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. |
||||
溶剤液(一定の濃度)を調合する
生物活性
| 製品説明 | Tolbutamide (HLS 831) is an inhibitor of potassium channel, used for type II diabetes. |
|---|---|
| in vitro | Tolbutamide belongs to a class of medications called sulfonylureas. Tolbutamide lowers blood sugar by causing the pancreas to produce insulin (a natural substance that is needed to break down sugar in the body) and helping the body use insulin efficiently. This medication will only help lower blood sugar in people whose bodies produce insulin naturally. Tolbutamide is not used to treat type 1 diabetes (condition in which the body does not produce insulin and, therefore, cannot control the amount of sugar in the blood) or diabetic ketoacidosis (a serious condition that may occur if high blood sugar is not treated). Tolbutamide inhibits both the basal and the cyclic AMP-stimulated protein kinase activities and the IC50 of Tolbutamide is 4 mM. Similar Tolbutamide concentrations are required for half maximal inhibition of in vitro lipolysis induced by hormones (norepinephrine and ACTH) or by dibutyryl cyclic AMP plus theophylline. Tolbutamide also inhibits both soluble and membrane-bound protein kinase from canine heart. The Tolbutamide inhibition of adipose tissue cyclic AMP-dependent protein kinase is one possible explanation for the antilipolytic effects of this drug. [1] Tolbutamide inhibits C6-glioma cell proliferation by increasing Cx43, which correlates with a reduction in pRb phosphorylation due to the up-regulation of the Cdk inhibitors p21 and p27. [2] Cytosolic nucelotides enhance the Tolbutamide sensitivity of the ATP-dependent K+ channel in mouse pancreatic B cells by their combined actions at inhibitory and stimulatory receptors. [3] Tolbutamide inhibits glucagon-induced phosphorylation of the bifunctional enzyme protein in a dose-dependent manner. By adding 2 mM Tolbutamide, reduces activity of 6PF-2-K and increased activity of Fru-2,6-P2ase in the presence of 10(-9) M glucagon are partially restored. The present results suggest the possibility that Tolbutamide modulates the activity of hepatic 6PF-2-K/Fru-2,6-P2ase through inhibiting a phosphorylation of the enzyme protein. [4] |
| in vivo | 450 mg Tolbutamide/kg/day given for 7 days significantly increases the binding of insulin to isolated adipocytes. The binding curves reflect an increase in the number of receptor sites rather than in the affinity. The effect is associated with an enhanced response to insulin of the adipose tissue, since the fat cells obtained from animals treated with Tolbutamide convert significantly more glucose to lipids in the presence of insulin than those obtained from the control group. However, the augmentation of insulin binding sites is observed only at a large tolbutamide dosage, which reduces the pancreatic insulin content, the secretory response of the isolated pancreas, and the serum insulin levels. Smaller doses, sufficient to produce metabolic effects via a stimulation of insulin secretion, do not provide additional insulin binding sites. [5] |
プロトコル(参考用のみ)
| キナーゼアッセイ | cAMP kinase assay | |
|---|---|---|
| Diced epididymal fat pads from fed Wistar rats (175-225 gm) are obtained after decapitation and incubated at 37 °C for two hours in Krebs-bicarbonate buffer containing 1.27 mM CaCl2. When added, Tolbutamide is present only during the incubation. After incubation fat pads are rinsed and sonicated in cold Krebs-bicarbonate buffer. The aqueous supematants from centrifugation at 50,000 × g for 30 minutes at 4 °C contained 0.75 to 1.25 mg protein per mL and are assayed for cyclic AMP-stimulated protein kinase activity. The assay is performed in 0.2 mL with these additions, 10 μmoles sodium glycerofiosphate pH 7.0, 2 μmoles sodium fluoride, 0.4 μmoles theophylline, 0.1 μmoles ethylene glyool bis (β-aminoethyl ether)-N, N'-tetraaoetic acid, 3 μmoles magnesium chloride, 0.3 mg mixed histone, 2 nmoles (γ- 32P) ATP, 1 nmoles cyclic AMP when indicated, and 0.05 ml of supernatant. | ||
| 細胞アッセイ | 細胞株 | C6 glioma cells |
| 濃度 | 400 μM | |
| 反応時間 | 24 hours | |
| 実験の流れ | C6 glioma cells are incubated in serum-free DMEM at 37 °C for at least 24 hours before each experiment. Tolbutamide (400 μM) is incubated for 24 hours in serum-free medium. Incubations are performed at 37 °C in an atmosphere of 95% air/5% CO2 with 90–95% humidity. | |
| 動物実験 | 動物モデル | Male albino Wistar rats (200-300 g) |
| 投薬量 | 450 mg/kg | |
| 投与方法 | Tolbutamide is administered orally for 7 days. | |
参考
Selleckの高級品が、幾つかの出版された研究調査結果(以下を含む)で使われた:
| Pump-Less, Recirculating Organ-On-Chip (rOoC) Platform To Model The Metabolic Crosstalk Between Islets and Liver [ Adv Healthc Mater, 2024, e2303785.] | PubMed: 38221504 |
| Establishment and Characterization of NCC-PMP1-C1: A Novel Patient-Derived Cell Line of Metastatic Pseudomyxoma Peritonei [ J Pers Med, 2022, 12(2)258] | PubMed: 35207746 |
| Establishment and characterization of NCC-UPS4-C1: a novel cell line of undifferentiated pleomorphic sarcoma from a patient with Li-Fraumeni syndrome [ Hum Cell, 2022, 10.1007/s13577-022-00671-y] | PubMed: 35118583 |
| Establishment and characterization of NCC-MFS4-C1: a novel patient-derived cell line of myxofibrosarcoma [ Hum Cell, 2021, 34(6):1911-1918] | PubMed: 34383271 |
| Establishment and characterization of the NCC-GCTB4-C1 cell line: a novel patient-derived cell line from giant cell tumor of bone [ Hum Cell, 2021, 10.1007/s13577-021-00639-4] | PubMed: 34731453 |
| Establishment and characterization of novel patient-derived cell lines from giant cell tumor of bone [ Hum Cell, 2021, 10.1007/s13577-021-00579-z] | PubMed: 34304386 |
| Establishment and characterization of patient-derived cancer models of malignant peripheral nerve sheath tumors. [ Cancer Cell Int, 2020, 19;20:58] | PubMed: 32099531 |
| Establishment and characterization of NCC-MFS2-C1: a novel patient-derived cancer cell line of myxofibrosarcoma [ Hum Cell, 2020, 10.1007/s13577-020-00420-z] | PubMed: 32870449 |
| Establishment and characterization of a novel cell line, NCC-TGCT1-C1, derived from a patient with tenosynovial giant cell tumor [ Hum Cell, 2020, 10.1007/s13577-020-00425-8] | PubMed: 32886306 |
長期の保管のために-20°Cの下で製品を保ってください。
人間や獣医の診断であるか治療的な使用のためにでない。
各々の製品のための特定の保管と取扱い情報は、製品データシートの上で示されます。大部分のSelleck製品は、推薦された状況の下で安定です。製品は、推薦された保管温度と異なる温度で、時々出荷されます。長期の保管のために必要とされてそれと異なる温度で、多くの製品は、短期もので安定です。品質を維持するが、夜通しの積荷のために最も経済的な貯蔵状況を用いてあなたの送料を保存する状況の下に、製品が出荷されることを、我々は確実とします。製品の受領と同時に、製品データシートの上で貯蔵推薦に従ってください。
