- 阻害剤
- 研究分野別
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- 化合物ライブラリー
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-Ⅱ
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at [email protected] to customize your library.
You could select:
- 抗体
- 新製品
- お問い合わせ
Telaprevir
別名:LY-570310, MP-424,VX-950
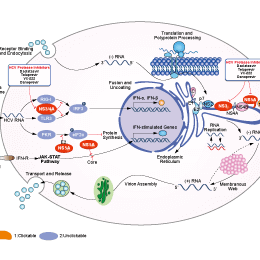
Telaprevir is an HCV NS3-4A serine protease inhibitor with IC50 of 0.35 μM.
CAS No. 402957-28-2
文献中Selleckの製品使用例(43)
製品安全説明書
現在のバッチを見る:
純度:
99.82%
99.82
Telaprevir関連製品
シグナル伝達経路
HCV Protease阻害剤の選択性比較
生物活性
| 製品説明 | Telaprevir is an HCV NS3-4A serine protease inhibitor with IC50 of 0.35 μM. | ||
|---|---|---|---|
| 特性 | Telaprevir is a covalent, reversible inhibitor of the NS3-4A protease (unlike BILN 2061which is a noncovalent inhibitor), with a slow-binding and slow-dissociation mechanism. | ||
| Targets |
|
| In Vitro | ||||
| In vitro | Telaprevir inhibits the hepatitis C virus NS3-4A serine protease, leading to the block of viral polyprotein processing and subsequently decrease of viral RNA replication, total HCV RNA levels and protein levels in the Con1 (genotype 1b) subgenomic HCV replicon cells in a time- and dose-dependent manner. Telaprevir displays a significant time-dependent increase in inhibitory effect on the replication of HCV RNA with IC50 values of 0.574 μM, 0.488 μM, 0.210 μM and 0.139 μM for 24, 48, 72 and 120 hours incubation, respectively. Telaprevir displays an average IC50 of 0.354 μM and an average IC90 of 0.830 μM, respectively, from three independent experiments using the 48 hours incubation. Telaprevir has no significant cytotoxicity to HCV replicon cells, parental Huh-7 and HepG2 cells after 48 hours incubation. Telaprevir (17.5 μM) completely eradicates HCV RNA from replicon cells after 13 days incubation without rebound after Telaprevir is withdrawn. Telaprevir displays an additive to moderate synergistic effect on reduction of HCV RNA replication and suppression of resistance mutations without significant increase in cytotoxicity when in combination with IFN-α, compared to treatment with each agent alone. [1] | |||
|---|---|---|---|---|
| Kinase Assay | Determination of anti-HCV activity | |||
| Stable Huh-7 cells containing the self-replicating, subgenomic HCV replicon, which is identical in sequence to the I377neo/NS3-3'/wt replicon are used for anti-HCV assays. Replicon cells are incubated at 37 °C for the indicated period of time with Telaprevir serially diluted in DMEM plus 2% FBS and 0.5% dimethyl sulfoxide (DMSO). Total cellular RNA is extracted using an RNeasy-96 kit, and the copy number of HCV RNA is determined using a quantitative RTPCR (QRT-PCR) assay for the assessment of 50% inhibitory concentration (IC50 | ||||
| 細胞実験 | 細胞株 | Huh-7, HepG2, and peripheral blood mononuclear cells (PBMC) | ||
| 濃度 | Dissolved in DMSO, final concentration ~1 mM | |||
| 反応時間 | 48 hours | |||
| 実験の流れ | Cells are incubated with various concentrations of Telaprevir for 48 hours. Cell viability is determined by using a tetrazolium (MTS)-based cell viability assay. | |||
| In Vivo | ||
| In Vivo | Oral administration of Telaprevir reduces HCV protease-dependent cleavage and subsequent secretion of SEAP from the liver into the blood in the mice model to 18.7% and 18.4% at dosage of 10 and 25 mg/kg, respectively. [2] Administration of Telaprevir at 200 mg/kg for 1 week results in a 1.9 log reduction of HCV RNA in genotype 1b HCV-infected human hepatocyte chimeric mice, and when treatment in combination with MK-0608 (50 mg/kg) for 4 weeks, viruses are eliminated from mice. [3] | |
|---|---|---|
| 動物実験 | 動物モデル | SCID mice injected with recombinant adenovirus (Ad-WT-HCVpro-SEAP or Ad-MT-HCVpro-SEAP) |
| 投与量 | ~300 mg/kg | |
| 投与経路 | Oral gavage | |
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT02881034 | Completed | Hepatitis C |
Hospices Civils de Lyon |
February 2014 | -- |
| NCT01994486 | Completed | Hepatitis C Chronic |
University of Florida|Vertex Pharmaceuticals Incorporated |
December 2013 | Phase 2 |
| NCT01980290 | Completed | Hepatitis Chronic |
Janssen Pharmaceutica N.V. Belgium |
May 2013 | -- |
| NCT01841502 | Terminated | Hepatitis C Infection|Depression |
Radboud University Medical Center|Janssen LP |
May 2013 | Phase 2 |
| NCT01766167 | Completed | Chronic Hepatitis C |
Mitsubishi Tanabe Pharma Corporation |
February 2013 | Phase 1 |
化学情報
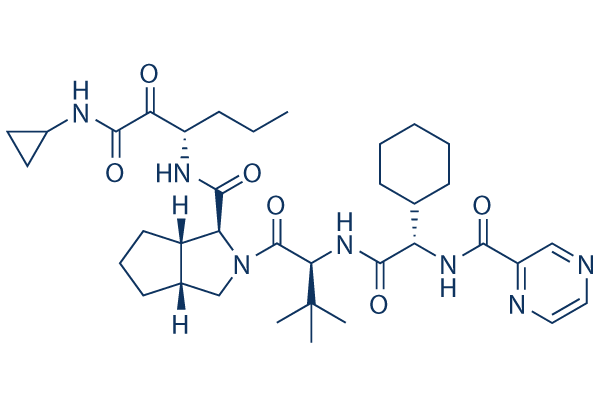
| 分子量 | 679.85 | 化学式 | C36H53N7O6 |
| CAS No. | 402957-28-2 | SDF | Download Telaprevir SDFをダウンロードする |
| Smiles | CCCC(C(=O)C(=O)NC1CC1)NC(=O)C2C3CCCC3CN2C(=O)C(C(C)(C)C)NC(=O)C(C4CCCCC4)NC(=O)C5=NC=CN=C5 | ||
| 保管 | |||
|
In vitro |
DMSO : 136 mg/mL ( (200.04 mM); 吸湿したDMSOは溶解度を減少させます。新しいDMSOをご使用ください。) Water : Insoluble Ethanol : Insoluble |
モル濃度計算器 |
|
in vivo Add solvents to the product individually and in order. |
投与溶液組成計算機 | ||||
実験計算
投与溶液組成計算機(クリア溶液)
ステップ1:実験データを入力してください。(実験操作によるロスを考慮し、動物数を1匹分多くして計算・調製することを推奨します)
mg/kg
g
μL
匹
ステップ2:投与溶媒の組成を入力してください。(ロット毎に適した溶解組成が異なる場合があります。詳細については弊社までお問い合わせください)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
計算結果:
投与溶媒濃度: mg/ml;
DMSOストック溶液調製方法: mg 試薬を μL DMSOに溶解する(濃度 mg/mL, 注:濃度が当該ロットのDMSO溶解度を超える場合はご連絡ください。 )
投与溶媒調製方法:Take μL DMSOストック溶液に μL PEG300,を加え、完全溶解後μL Tween 80,を加えて完全溶解させた後 μL ddH2O,を加え完全に溶解させます。
投与溶媒調製方法:μL DMSOストック溶液に μL Corn oil,を加え、完全溶解。
注意:1.ストック溶液に沈殿、混濁などがないことをご確認ください;
2.順番通りに溶剤を加えてください。次のステップに進む前に溶液に沈殿、混濁などがないことを確認してから加えてください。ボルテックス、ソニケーション、水浴加熱など物理的な方法で溶解を早めることは可能です。
技術サポート
ストックの作り方、阻害剤の保管方法、細胞実験や動物実験の際に注意すべき点など、製品を取扱う時に問い合わせが多かった質問に対しては取扱説明書でお答えしています。
他に質問がある場合は、お気軽にお問い合わせください。
* 必須
Tags: Telaprevirを買う | Telaprevir ic50 | Telaprevir供給者 | Telaprevirを購入する | Telaprevir費用 | Telaprevir生産者 | オーダーTelaprevir | Telaprevir化学構造 | Telaprevir分子量 | Telaprevir代理店