- 阻害剤
- 研究分野別
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- 化合物ライブラリー
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-Ⅱ
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at [email protected] to customize your library.
You could select:
- 抗体
- 新製品
- お問い合わせ
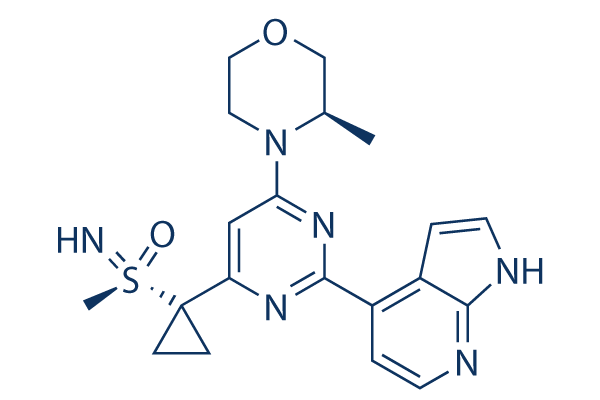
Ceralasertib (AZD6738)
Ceralasertib (AZD6738) is an orally active, and selective ATR kinase inhibitor with IC50 of 1 nM. Phase 1/2.
CAS No. 1352226-88-0
文献中Selleckの製品使用例(104)
製品安全説明書
現在のバッチを見る:
純度:
99.64%
99.64
Ceralasertib (AZD6738)と併用されることが多い化合物
Ceralasertib and Durvalumab combination has promising antitumor activity, with durable responses in patients with refractory advanced gastric cancer (AGC).
Ceralasertib and Olaparib combination is undergoing a phase II study for metastatic triple-negative breast cancer (TNBC).
Ceralasertib and Carboplatin combination demonstrate excellent anticancer potential in phase I studies in cancer patients.
Huang X, et al. J Enzyme Inhib Med Chem. 2023 Dec;38(1):2237209.
Ceralasertib and Paclitaxel combination demonstrate excellent anticancer potential in phase I studies in cancer patients.
Huang X, et al. J Enzyme Inhib Med Chem. 2023 Dec;38(1):2237209.
Ceralasertib and Adavosertib combination exert more potent anti-tumor effects against biliary tract cancer.
Ceralasertib (AZD6738)関連製品
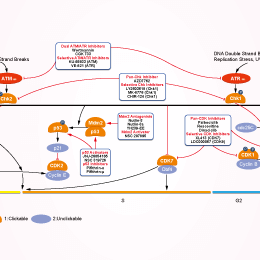
シグナル伝達経路
ATM/ATR阻害剤の選択性比較
Cell Data
| Cell Lines | Assay Type | Concentration | Incubation Time | 活性情報 | PMID |
|---|---|---|---|---|---|
| breast cancer cell lines | Cell growth inhibition assay | 0.125, 0.25, 0.5 and 1.0 μM | 5 days | IC50 values ranged from 0.3 to >1 μmol/L | 27501113 |
| SNU-601 cells | Cell growth inhibition assay | 0-1 μmol/L | 5 days | The S and sub-G1 populations of SNU-601 cells were dramatically and dose-dependently increased by AZD6738. | 28138034 |
| K8484 cells | Function assay | 2 μM | 7 hours | In K8484 cells, AZD6738 at 2 µM completely prevented gemcitabine-induced Chk1 phosphorylation on Serine 345, the downstream ATR target. | 29891488 |
| LoVo cells | Function assay | 25 mg/kg | 8 hrs | Cp = 0.74 μM | 30346772 |
| LoVo cells | Function assay | 50 mg/kg | 8 hrs | Cp = 2.2 μM | 30346772 |
| LoVo cells | Function assay | 75 mg/kg | 8 hrs | Cp = 2.6 μM | 30346772 |
| LICR-LON-HN4 and LICR-LON-HN5 cells | Function assay | 0.03, 0.1, 0.3, 1, 3, 10 μM | AZD6738 inhibition of ATR through loss of downstream phosphorylation of CHK1 on Ser345. | 30057890 | |
| LoVo cells | Function assay | 24 h | Reduction in cell count; a proportion of the cell population are (in addition to cell cycle arrest) undergoing apoptosis when exposed to drug at concentrations greater than 3 μM | 26310312 | |
| HT29 cells | Function assay | 60 mins | IC50 = 0.074 μM | 30346772 | |
| LoVo cells | Cytotoxicity assay | 72 hrs | GI50 = 0.44 μM | 30346772 | |
| HT-29 cells | Cytotoxicity assay | 72 hrs | GI50 = 2.6 μM | 30346772 | |
| MDA-MB-468 cells | Function assay | IC50 = 5.7 μM | 30346772 | ||
| 他の多くの細胞株試験データをご覧になる場合はこちらをクリックして下さい | |||||
生物活性
| 製品説明 | Ceralasertib (AZD6738) is an orally active, and selective ATR kinase inhibitor with IC50 of 1 nM. Phase 1/2. | ||
|---|---|---|---|
| Targets |
|
| In Vitro | ||||
| In vitro | In four Kras mutant cell lines: H23, H460, A549, and H358, AZD6738 inhibits ATR kinase activity and impairs cell viability. In ATM-deficient H23 cells, AZD6738 strongly synergizes with cisplatin to induce rapid cell death. [1] In p53 or ATM defective cells, AZD6738 treatment results in replication fork stalls and accumulation of unrepaired DNA damage, resulting in cell death by mitotic catastrophe. [2] | |||
|---|---|---|---|---|
| 細胞実験 | 細胞株 | H23, H460, A549, and H358 cells | ||
| 濃度 | ~30 μM | |||
| 反応時間 | 48 h | |||
| 実験の流れ | Cells are treated in white walled, clear bottom 96-well plates with the indicated doses of AZD6738, cisplatin, gemcitabine, or combination for 48 h. ATP levels are assessed as surrogate measure of viability is assessed using the CellTiter-Glo Luminescent Cell Viability Assay and Safire2 plate reader. Raw data are corrected for background luminescence prior to further analysis. For AZD6738 treatment, log dose response curves are generated in GraphPad Prism 6 by nonlinear regression (log(inhibitor) vs. response with variable slope) of log-transformed (x = log(x)) data normalized to the mean of untreated controls. GI50 values, defined as the dose X at which Y = 50%, were extrapolated from dose response curves. | |||
| 実験結果図 | Methods | Biomarkers | 結果図 | PMID |
| Western blot | ATM pSer1981 / ATM / ATR / Chk1 pSer345 / Chk1 / Chk2 pThr68 / Chk2 pCHK1 / pCDC25c / pRPA32 / γH2AX / pHH3 / cleaved caspase-3 / RAD51 |
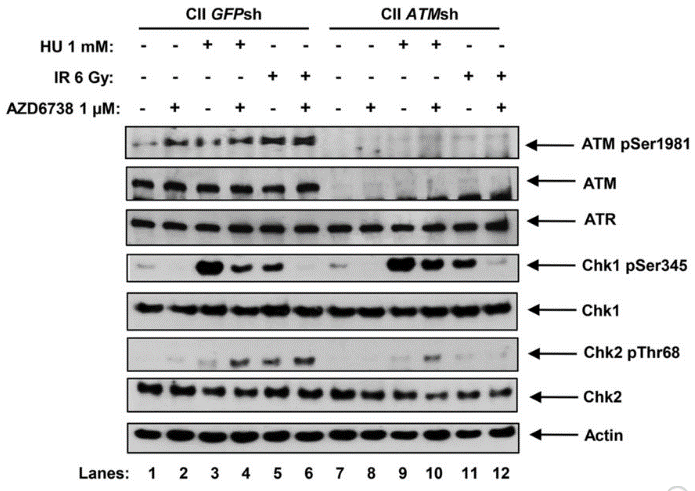
|
26563132 | |
| Immunofluorescence | 53BP1 γH2AX / RAD51 |
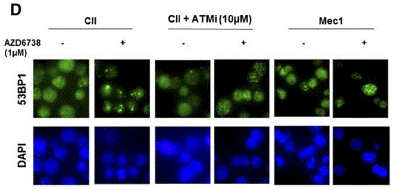
|
26563132 | |
| Growth inhibition assay | IC50 Cell viability |
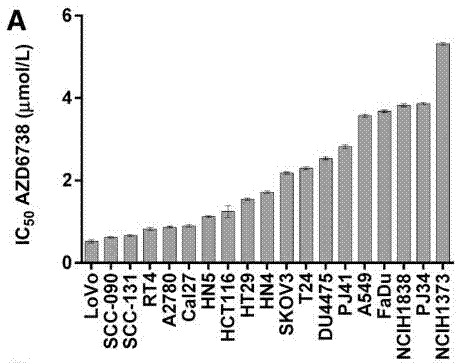
|
28062704 | |
| In Vivo | ||
| In Vivo | In nude mice bearing H460 and H23 tumors, AZD6738 (50 mg/kg, p.o.) results in tumor growth inhibition (TGI), and the the combination with cisplatin causes rapid regression of ATM-deficient H23 tumors. [1] In nude mice bearing LoVo xenografts, a combination of AZD6738 (50 mg/kg) + IR (2 Gy) avoids toxicity while still maintaining efficacy. [3] | |
|---|---|---|
| 動物実験 | 動物モデル | Female athymic nude mice bearing H23 or H460 xenografts |
| 投与量 | 25 or 50 mg/kg | |
| 投与経路 | p.o. | |
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05941897 | Recruiting | Advanced or Metastatic NSCLC |
AstraZeneca |
June 21 2023 | Phase 2 |
| NCT05514132 | Active not recruiting | Advanced Solid Tumours |
AstraZeneca |
September 23 2022 | Phase 1 |
| NCT05450692 | Recruiting | Advanced or Metastatic Non-Small Cell Lung Cancer |
AstraZeneca|Parexel |
September 15 2022 | Phase 3 |
| NCT05061134 | Active not recruiting | Melanoma |
AstraZeneca |
August 11 2022 | Phase 2 |
| NCT05469919 | Active not recruiting | Advanced Solid Malignancies |
AstraZeneca |
June 9 2022 | Phase 1 |
| NCT04704661 | Recruiting | Advanced Breast Carcinoma|Advanced Colon Carcinoma|Advanced Colorectal Carcinoma|Advanced Endometrial Carcinoma|Advanced Gastric Carcinoma|Advanced Gastroesophageal Junction Adenocarcinoma|Advanced Malignant Solid Neoplasm|Advanced Salivary Gland Carcinoma|Anatomic Stage III Breast Cancer AJCC v8|Anatomic Stage IIIA Breast Cancer AJCC v8|Anatomic Stage IIIB Breast Cancer AJCC v8|Anatomic Stage IIIC Breast Cancer AJCC v8|Anatomic Stage IV Breast Cancer AJCC v8|Clinical Stage III Gastric Cancer AJCC v8|Clinical Stage III Gastroesophageal Junction Adenocarcinoma AJCC v8|Clinical Stage IV Gastric Cancer AJCC v8|Clinical Stage IV Gastroesophageal Junction Adenocarcinoma AJCC v8|Clinical Stage IVA Gastric Cancer AJCC v8|Clinical Stage IVA Gastroesophageal Junction Adenocarcinoma AJCC v8|Clinical Stage IVB Gastric Cancer AJCC v8|Clinical Stage IVB Gastroesophageal Junction Adenocarcinoma AJCC v8|HER2-Positive Breast Carcinoma|Malignant Hepatobiliary Neoplasm|Metastatic Breast Carcinoma|Metastatic Gastroesophageal Junction Adenocarcinoma|Metastatic Malignant Solid Neoplasm|Pathologic Stage III Gastric Cancer AJCC v8|Pathologic Stage III Gastroesophageal Junction Adenocarcinoma AJCC v8|Pathologic Stage IIIA Gastric Cancer AJCC v8|Pathologic Stage IIIA Gastroesophageal Junction Adenocarcinoma AJCC v8|Pathologic Stage IIIB Gastric Cancer AJCC v8|Pathologic Stage IIIB Gastroesophageal Junction Adenocarcinoma AJCC v8|Pathologic Stage IIIC Gastric Cancer AJCC v8|Pathologic Stage IV Gastric Cancer AJCC v8|Pathologic Stage IV Gastroesophageal Junction Adenocarcinoma AJCC v8|Pathologic Stage IVA Gastroesophageal Junction Adenocarcinoma AJCC v8|Pathologic Stage IVB Gastroesophageal Junction Adenocarcinoma AJCC v8|Prognostic Stage III Breast Cancer AJCC v8|Prognostic Stage IIIA Breast Cancer AJCC v8|Prognostic Stage IIIB Breast Cancer AJCC v8|Prognostic Stage IIIC Breast Cancer AJCC v8|Prognostic Stage IV Breast Cancer AJCC v8|Stage III Colon Cancer AJCC v8|Stage III Colorectal Cancer AJCC v8|Stage III Major Salivary Gland Cancer AJCC v8|Stage III Uterine Corpus Cancer AJCC v8|Stage IIIA Colon Cancer AJCC v8|Stage IIIA Colorectal Cancer AJCC v8|Stage IIIA Uterine Corpus Cancer AJCC v8|Stage IIIB Colon Cancer AJCC v8|Stage IIIB Colorectal Cancer AJCC v8|Stage IIIB Uterine Corpus Cancer AJCC v8|Stage IIIC Colon Cancer AJCC v8|Stage IIIC Colorectal Cancer AJCC v8|Stage IIIC Uterine Corpus Cancer AJCC v8|Stage IIIC1 Uterine Corpus Cancer AJCC v8|Stage IIIC2 Uterine Corpus Cancer AJCC v8|Stage IV Colon Cancer AJCC v8|Stage IV Colorectal Cancer AJCC v8|Stage IV Major Salivary Gland Cancer AJCC v8|Stage IV Uterine Corpus Cancer AJCC v8|Stage IVA Colon Cancer AJCC v8|Stage IVA Colorectal Cancer AJCC v8|Stage IVA Major Salivary Gland Cancer AJCC v8|Stage IVA Uterine Corpus Cancer AJCC v8|Stage IVB Colon Cancer AJCC v8|Stage IVB Colorectal Cancer AJCC v8|Stage IVB Major Salivary Gland Cancer AJCC v8|Stage IVB Uterine Corpus Cancer AJCC v8|Stage IVC Colon Cancer AJCC v8|Stage IVC Colorectal Cancer AJCC v8|Stage IVC Major Salivary Gland Cancer AJCC v8|Unresectable Colorectal Carcinoma|Unresectable Gastroesophageal Junction Adenocarcinoma|Unresectable Malignant Solid Neoplasm |
National Cancer Institute (NCI) |
August 9 2021 | Phase 1 |
化学情報
| 分子量 | 412.51 | 化学式 | C20H24N6O2S |
| CAS No. | 1352226-88-0 | SDF | Download Ceralasertib (AZD6738) SDFをダウンロードする |
| Smiles | CC1COCCN1C2=NC(=NC(=C2)C3(CC3)S(=N)(=O)C)C4=C5C=CNC5=NC=C4 | ||
| 保管 | |||
|
In vitro |
DMSO : 83 mg/mL ( (201.2 mM); 吸湿したDMSOは溶解度を減少させます。新しいDMSOをご使用ください。) Ethanol : 83 mg/mL Water : Insoluble |
モル濃度計算器 |
|
in vivo Add solvents to the product individually and in order. |
投与溶液組成計算機 | ||||
実験計算
投与溶液組成計算機(クリア溶液)
ステップ1:実験データを入力してください。(実験操作によるロスを考慮し、動物数を1匹分多くして計算・調製することを推奨します)
mg/kg
g
μL
匹
ステップ2:投与溶媒の組成を入力してください。(ロット毎に適した溶解組成が異なる場合があります。詳細については弊社までお問い合わせください)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
計算結果:
投与溶媒濃度: mg/ml;
DMSOストック溶液調製方法: mg 試薬を μL DMSOに溶解する(濃度 mg/mL, 注:濃度が当該ロットのDMSO溶解度を超える場合はご連絡ください。 )
投与溶媒調製方法:Take μL DMSOストック溶液に μL PEG300,を加え、完全溶解後μL Tween 80,を加えて完全溶解させた後 μL ddH2O,を加え完全に溶解させます。
投与溶媒調製方法:μL DMSOストック溶液に μL Corn oil,を加え、完全溶解。
注意:1.ストック溶液に沈殿、混濁などがないことをご確認ください;
2.順番通りに溶剤を加えてください。次のステップに進む前に溶液に沈殿、混濁などがないことを確認してから加えてください。ボルテックス、ソニケーション、水浴加熱など物理的な方法で溶解を早めることは可能です。
技術サポート
ストックの作り方、阻害剤の保管方法、細胞実験や動物実験の際に注意すべき点など、製品を取扱う時に問い合わせが多かった質問に対しては取扱説明書でお答えしています。
他に質問がある場合は、お気軽にお問い合わせください。
* 必須
Tags: Ceralasertib (AZD6738)を買う | Ceralasertib (AZD6738) ic50 | Ceralasertib (AZD6738)供給者 | Ceralasertib (AZD6738)を購入する | Ceralasertib (AZD6738)費用 | Ceralasertib (AZD6738)生産者 | オーダーCeralasertib (AZD6738) | Ceralasertib (AZD6738)化学構造 | Ceralasertib (AZD6738)分子量 | Ceralasertib (AZD6738)代理店