|
受注:045-509-1970 |
技術サポート:tech@selleck.co.jp 平日9:00〜18:00 1営業日以内にご連絡を差し上げます |
化学情報
|
Synonyms | N/A | Storage (From the date of receipt) |
3 years-20°C powder | |||||||||||
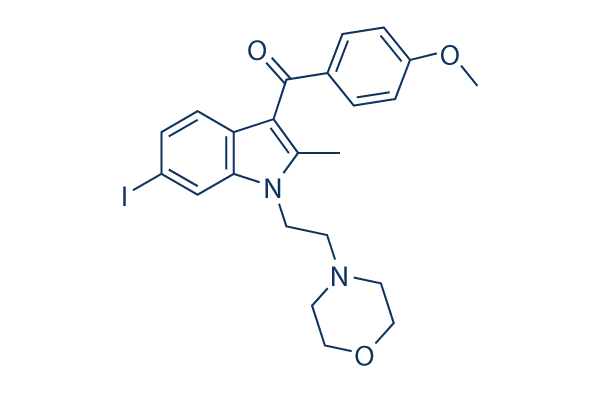
| 化学式 | C23H25IN2O3 |
||||||||||||||
| 分子量 | 504.36 | CAS No. | 164178-33-0 | ||||||||||||
| Solubility (25°C)* | 体外 | DMSO | 50 mg/mL (99.13 mM) | ||||||||||||
| Water | Insoluble | ||||||||||||||
| Ethanol | Insoluble | ||||||||||||||
| 体内 (毎回新しく調製した物を用意してください) |
|
||||||||||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. |
|||||||||||||||
溶剤液(一定の濃度)を調合する
生物活性
| 製品説明 | 6-Iodopravadoline (AM630)は、Kiが31.2 nMの選択的カンナビノイドCB2受容体アンタゴニストです。 |
|---|---|
| in vitro | AM630 (6-Iodopravadoline) is a CB2 cannabinoid receptor ligand with Ki of 31.2 nM and a CB2 /CB1 affinity ratio of 165. It inhibits [35 S]-GTPγS binding to CB2 receptor membranes (EC50=76.6 nM), enhances forskolin-stimulated cyclic AMP production in CB2-transfected cells (5.2 fold by 1 μM ), and antagonizes the inhibition of forskolin-stimulated cyclic AMP production in this cell line induced by CP55940. This compound (10 μM) inhibits forskolin-stimulated cyclic AMP production In CB1-transfected cells by 45.9%. It behaves as a competitive antagonist of △9-THC, CP 55,940, WIN 55212-2, anandamide and (R)-(+)-arachidonyl-l’-hydroxy-2’-propylamide (AM356) in mouse isolated vas deferens with Kd of 14.0, 17.3, 36.5, 278.8, and 85.9 nM, respectively. At 10 μM, it activates a robust Ca2+ accumulation in a subset (35- 40%) of TG neurons, and with EC50 of 15.6 μM. This compound is able to generate currents in TG sensory neurons with the activation threshold of 1 μM. Its responses are mediated by the TRPA1 channel in a majority of TG small-to-medium sensory neurons, which is modulated by TRPV1. Pre-treatment with AM630 (25 μM) is able to inhibits the Capsaicin (CAP) effects, mustard oil (MO) and WIN 55,212-2 (WIN) TRPA1 mediated responses. At 100 nM, it effectively inhibits osteoclastogenesis in culture with RANKL in the presence and absence of Ti particles, as reducing the number of tartrate-resistant acid phosphatase-positive cells by more than 50%. This compound (100 nM) inhibits mRNA expression of RANK and cathepsin K in RAW cells stimulated by Ti particles and RANKL. It (100 nM) reduces protein expression of interleukin-1β and tumor necrosis factor-α in RAW cells cultured with Ti particles. AM630 has no toxic effect on RAW cells. |
| in vivo | 6-Iodopravadoline (AM630) (30 μg) injection is not able to induce nociceptive behaviors or thermal hyperalgesia in hind paw of WT mice, but significantly attenuates CAP-induced thermal hyperalgesia. This compound (30 μg) is able to reverse WIN inhibition of CAP-induced thermal hyperalgesia. It exerts its peripheral effects by not only inhibiting CB1 and CB2, but also activating TRPA1 channels and subsequently desensitizing TRPA1 as well as TRPV1 channels. |
プロトコル(参考用のみ)
| キナーゼアッセイ | [35 S]-GTPγS binding assay | |
|---|---|---|
| CB2 transfected cells are removed from flasks by scraping, resuspended in homogenization buffer (0.32 M sucrose and 50 mM Tris), and homogenized using an ultra-Turrex homogenizer. The homogenate is diluted with Tris buffer (50 mM, pH 7.4) and centrifuged at 50,000 g for 45 min. Cell membranes (20 μg) are incubated in assay buffer containing 2 mg/mL fatty acid free BSA, 20 μM GDP and 0.1 nM 35 S]-GTPγS. The assay buffer containes 50 mM Tris, 10 mM MgCl2, 100 mM NaCl and 0.2 mM EDTA at pH 7.4. Incubations are carried out at 30 ℃ for 90 min in a total volume of 500 μL. The reaction is terminated by the addition of 4 mL of ice-cold wash buffer (50 mM Tris and 1 mg/mL BSA, pH 7.4) followed by rapid filtration under vacuum through Whatman GF/B glass-fibre filters (pre-soaked in wash buffer) using a 12-tube Brandel cell harvester. The tubes are washed three times with 4 mL of wash buffer. Filters are oven dried, placed in 5 mL of scintillation fluid and bound radioactivity is determined by liquid scintillation counting. Basal binding of 35 S]-GTPγS is determined in the presence of 20 μM GDP and absence of cannabinoid. Non-specific binding is determined in the presence of 10 μM GTPγS. | ||
参考
|
Selleckの高級品が、幾つかの出版された研究調査結果(以下を含む)で使われた:
| CB2R activation ameliorates late adolescent chronic alcohol exposure-induced anxiety-like behaviors during withdrawal by preventing morphological changes and suppressing NLRP3 inflammasome activation in prefrontal cortex microglia in mice [ Brain Behav Immun, 2023, 110:60-79] | PubMed: 36754245 |
| Wwl70-induced ABHD6 inhibition attenuates memory deficits and pathological phenotypes in APPswe/PS1dE9 mice [ Pharmacol Res, 2023, 194:106864] | PubMed: 37480972 |
長期の保管のために-20°Cの下で製品を保ってください。
人間や獣医の診断であるか治療的な使用のためにでない。
各々の製品のための特定の保管と取扱い情報は、製品データシートの上で示されます。大部分のSelleck製品は、推薦された状況の下で安定です。製品は、推薦された保管温度と異なる温度で、時々出荷されます。長期の保管のために必要とされてそれと異なる温度で、多くの製品は、短期もので安定です。品質を維持するが、夜通しの積荷のために最も経済的な貯蔵状況を用いてあなたの送料を保存する状況の下に、製品が出荷されることを、我々は確実とします。製品の受領と同時に、製品データシートの上で貯蔵推薦に従ってください。
