|
受注:045-509-1970 |
技術サポート:tech@selleck.co.jp 平日9:00〜18:00 1営業日以内にご連絡を差し上げます |
化学情報
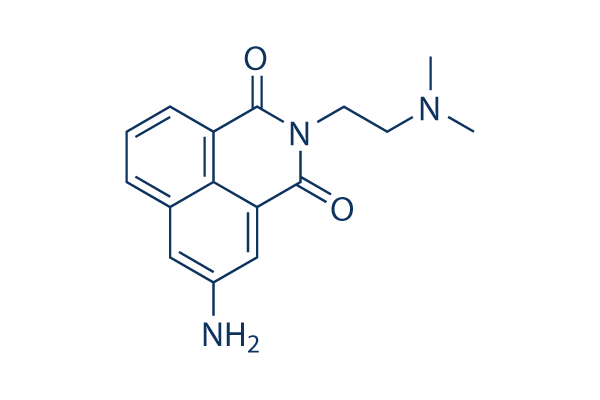
|
Synonyms | NSC308847,AS1413 | Storage (From the date of receipt) |
3 years -20°C powder 1 years -80°C in solvent |
|||
| 化学式 | C16H17N3O2 |
||||||
| 分子量 | 283.33 | CAS No. | 69408-81-7 | ||||
| Solubility (25°C)* | 体外 | DMSO | 57 mg/mL (201.17 mM) | ||||
| Ethanol | 4 mg/mL (14.11 mM) | ||||||
| Water | Insoluble | ||||||
| 体内 (毎回新しく調製した物を用意してください) |
|
||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. |
|||||||
溶剤液(一定の濃度)を調合する
生物活性
| 製品説明 | Amonafide (NSC308847, AS1413) produces protein-associated DNA-strand breaks through a topoisomerase II-mediated reaction, but does not produce topoisomerase I-mediated DNA cleavage. Phase 3. |
|---|---|
| in vitro | Through a topoisomerase II-mediated reaction, Amonafide treatment produces DNA single-strand breaks (SSB), double-strand breaks (DSB), and DNA-protein cross-links in human myeloid leukemia cells. This compound treatment inhibits colony formation of the leukemic cell lines and the normal human bone marrow GM-CFC in a dose-dependent manner. It does not produce topoisomerase I-mediated DNA cleavage even at 100 μM. The m-AMSA-resistant line is less than 2-fold resistant to this compound [1] This chemical interferes with the DNA breakage-reunion activity of mammalian DNA topoisomerase II resulting in DNA cleavage stimulation. [2] Compared with those of other antitumor drugs, this compound-stimulated cleavage intensity patterns are markedly different. It highly prefers a cytosine, and excludes guanines and thymines instead, at position -1, with lower preference for an adenine at position +1. [3] Topoisomerase II-mediated DNA cleavage induced by this agent is affected only slightly (less than 3-fold) by 1 mM ATP, suggesting that it is an ATP-insensitive topoisomerase II inhibitor in contrast to doxorubicin, etoposide, and mitoxantrone. [4] This compound significantly inhibits the growth of HT-29, HeLa, and PC3 cells with IC50 of 4.67 μM, 2.73 μM, and 6.38 μM, respectively. [5] It is unaffected by P-glycoprotein-mediated efflux, unlike those of the classical topoisomerase II inhibitors (daunorubicin, doxorubicin, idarubicin, etoposide, and mitoxantrone). [6] |
プロトコル(参考用のみ)
| 細胞アッセイ | 細胞株 | HT-29, HeLa, and PC3 |
|---|---|---|
| 濃度 | Dissolved in DMSO, final concentrations ~10 μM | |
| 反応時間 | 72 hours | |
| 実験の流れ | All cell lines are in the logarithmic phase of growth when the assay of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) is carried out. Cells are harvested and seeded into 96-well tissue culture plates at a density of 2.5 × 103 cells/well in 150 μL aliquots of medium. The concentrations tested are serial dilutions of a stock solution (10 μM in DMSO) with phosphate-buffered saline (PBS) and are added 24 hours later. The assay is ended after 72 hours of this compound exposure and PBS is used as a negative control. After 72 hours treatment, cells are washed twice with PBS, and then 50 μL/well of MTT reagent (1 mg/mL in PBS) together with 150 μL/well of prewarmed medium are added. The plates are returned to the incubator for 4 hours. Subsequently, DMSO is added as solvent. Absorbance is determined at 570 nm with a Microplate reader. All experiments are performed at least three times, and the average of the percentage absorbance is plotted against concentration. Then, the concentration of this chemical required to inhibit 50% of cell growth (IC50) is calculated for it. |
参考
|
Selleckの高級品が、幾つかの出版された研究調査結果(以下を含む)で使われた:
| Patient-derived rhabdomyosarcoma cells recapitulate the genetic and transcriptomic landscapes of primary tumors [ iScience, 2024, 27(10):110862] | PubMed: 39319271 |
| Topoisomerase II as a Novel Antiviral Target against Panarenaviral Diseases [ Viruses, 2022, 15(1)105] | PubMed: 36680145 |
| Repurposing DNA-binding agents as H-bonded organic semiconductors. [ Nat Commun, 2019, 10(1):4217] | PubMed: 31527590 |
| Inhibition of Ubiquitin Specific Protease 1 Sensitizes Colorectal Cancer Cells to DNA-Damaging Chemotherapeutics. [ Front Oncol, 2019, 9:1406] | PubMed: 31921663 |
| MRI visible drug eluting magnetic microspheres for transcatheter intra-arterial delivery to liver tumors. [ Theranostics, 2015, 5(5):477-88] | PubMed: 25767615 |
| MRI Visible Drug Eluting Magnetic Microspheres for Transcatheter Intra-Arterial Delivery to Liver Tumors. [Kim DH, et al. Theranostics, 2015, 5(5): 477-488] | |
| Antiproliferative and apoptosis-inducing activities of novel naphthalimide–cyclam conjugates through dual topoisomerase (topo) I/II inhibition [Tan S, et al. Bioorg Med Chem, 2015, 23(17):5672-80] | PubMed: 26211460 |
| Bioluminescent cell-based NAD(P)/NAD(P)H assays for rapid dinucleotide measurement and inhibitor screening [ Assay Drug Dev Technol, 2014, 12(9-10):514-26] | PubMed: 25506801 |
長期の保管のために-20°Cの下で製品を保ってください。
人間や獣医の診断であるか治療的な使用のためにでない。
各々の製品のための特定の保管と取扱い情報は、製品データシートの上で示されます。大部分のSelleck製品は、推薦された状況の下で安定です。製品は、推薦された保管温度と異なる温度で、時々出荷されます。長期の保管のために必要とされてそれと異なる温度で、多くの製品は、短期もので安定です。品質を維持するが、夜通しの積荷のために最も経済的な貯蔵状況を用いてあなたの送料を保存する状況の下に、製品が出荷されることを、我々は確実とします。製品の受領と同時に、製品データシートの上で貯蔵推薦に従ってください。
