|
受注:045-509-1970 |
技術サポート:tech@selleck.co.jp 平日9:00〜18:00 1営業日以内にご連絡を差し上げます |
化学情報
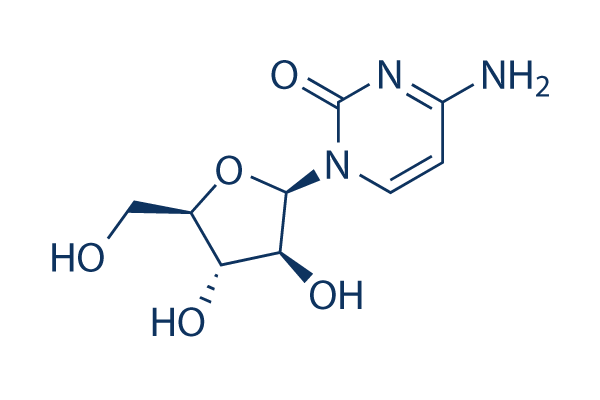
|
Synonyms | Cytarabin,Ara-C,Arabinofuranosyl Cytidine,Cytosine β-D-arabinofuranoside,Cytosine arabinoside,NSC 63878,NSC 287459,U-19920A | Storage (From the date of receipt) |
3 years -20°C powder 1 years -80°C in solvent |
|||||||
| 化学式 | C9H13N3O5 |
||||||||||
| 分子量 | 243.22 | CAS No. | 147-94-4 | ||||||||
| Solubility (25°C)* | 体外 | DMSO | 49 mg/mL (201.46 mM) | ||||||||
| Water | 49 mg/mL (201.46 mM) | ||||||||||
| Ethanol | Insoluble | ||||||||||
| 体内 (毎回新しく調製した物を用意してください) |
|
||||||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. |
|||||||||||
溶剤液(一定の濃度)を調合する
生物活性
| 製品説明 | Cytarabine is an antimetabolic agent and DNA synthesis inhibitor with IC50 of 16 nM in wild-type CCRF-CEM cells. Cytarabine induces autophagy and apoptosis. |
|---|---|
| in vitro | Cytarabine (AraC) is phosphorylated into a triphosphate form (Ara-CTP) involving deoxycytidine kinase (dCK), which competes with dCTP for incorporation into DNA, and then blocks DNA synthesis by inhibiting the function of DNA and RNA polymerases. This compound displays a higher growth inhibitory activity towards wild-type CCRF-CEM cells compared to other acute myelogenous leukemia (AML) cells with IC50 of 16 nM. [1] Increasing concentrations of this chemical (IC50 of 0.69 μM) results in decreased metabolic activity of sensitive rat leukemic cell line RO/1, and the cell toxity can be highly enhanced by transfection with human wt dCK (IC50 of 0.037 μM) but not the inactive, alternatively spliced dCK forms. [2] It apparently induces apoptosis of rat sympathetic neurons at 10 μM, of which 100 μM shows the highest toxicity and kills over 80% of the neurons by 84 hours, involving the release of mitochondrial cytochrome-c and the activation of caspase-3, and the toxicity can be attenuated by p53 knockdown and delayed by bax deletion. [3] |
| in vivo | Cytarabine is highly effective against acute leukaemias, which causes the characteristic G1/S blockage and synchronization, and increases the survival time for leukaemic Brown Norway rats in a weak dose-related fashion indicating that the use of higher dosages of this compound does not contribute to its antileukaemic effectiveness in man. [4] This chemical also causes placental growth retardation and increases placental trophoblastic cells apoptosis in the placental labyrinth zone of the pregnant Slc:Wistar rats, which increases from 3 hour after the treatment and peaks at 6 hour before returning to control levels at 48 hour, with remarkably enhanced p53 protein, p53 trancriptional target genes such as p21, cyclinG1 and fas and caspase-3 activity. [5] |
| 特徴 | The 1st of a series of cancer drugs that alters the sugar component of nucleosides. |
プロトコル(参考用のみ)
| キナーゼアッセイ | In Vitro Growth Inhibition Assay | |
|---|---|---|
| Stock solution of Cytarabine is prepared in absolute ethanol, and serial dilutions of this compound are prepared. CCRF-CEM cells are suspended in RPMI medium supplemented with 10% FBS, 0.1% gentamicin, and 1% sodium pyruvate. The cells are suspended in their respective media to give 10 mL volumes of cell suspension at a final density of 3-6 × 104 cells/mL. Appropriate volumes of this chemical solution are transferred to the cell suspensions, and incubation is continued for 72 hours. The cells are spun down and resuspended in fresh this compound -free medium, and final cell counts are determined. The data are analyzed by sigmoidal curve fitting of the cell count versus this chemical concentration, and the results are expressed as the IC50 (this compound concentration that inhibits cell growth to 50% of the control value). | ||
| 細胞アッセイ | 細胞株 | Rat leukemic cell lines RCL/0, RO/1 and K7 and human myelomonocytic leukemic U937 |
| 濃度 | ~100 μM | |
| 反応時間 | 24, 48 and 72 hours | |
| 実験の流れ | Cells are incubated in the presence of different concentrations of Cytarabine at 37 °C for 24, 48, and 72 hours. At the time of 20-, 44-, or 68-hour incubation in the presence of this compound, 10 mL of cell proliferation reagent WST-1 solution is added. After 2- and 4-hour incubation with WST-1, cell metabolic activity is assessed with colorimetric changes quantified by measuring the absorbance in a spectrophotometer at 450 nm. And cell division times are calculated from eosin counting in parallel with viability assay |
|
| 動物実験 | 動物モデル | Brown Norway rat with myelocytic leukaemia |
| 投薬量 | 5 - 1000 mg/kg | |
| 投与方法 | Injection i.v. | |
参考
|
カスタマーフィードバック
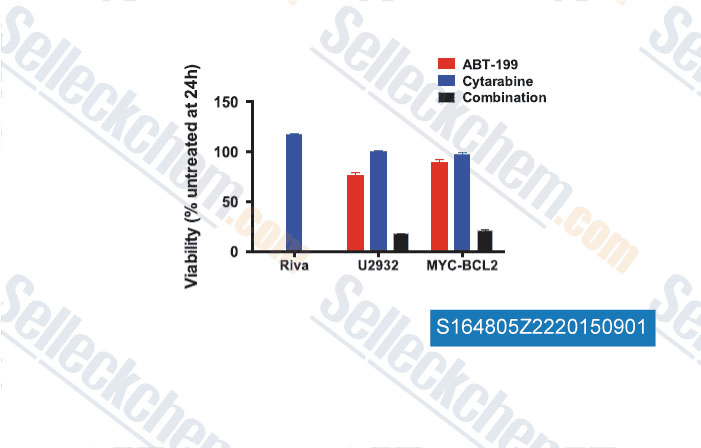
-
Data from [Data independently produced by , , Leukemia, 2015, 29(8): 1702–1712]
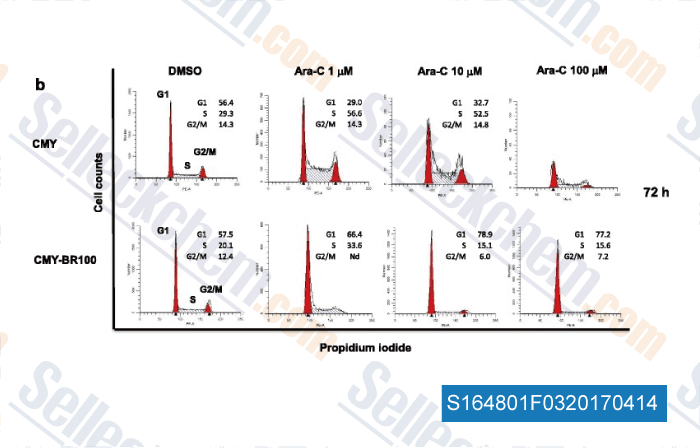
-
Data from [Data independently produced by , , J Exp Clin Cancer Res, 2017, 36(1):22]
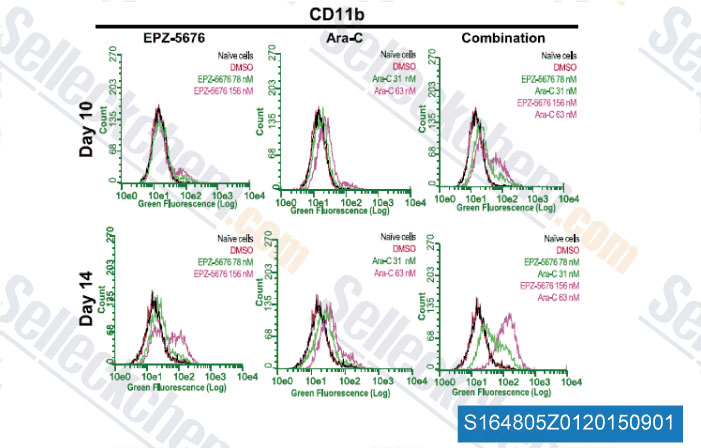
-
Data from [Data independently produced by , , J Pharmacol Exp Ther, 2014, 350(3): 646-56]
Selleckの高級品が、幾つかの出版された研究調査結果(以下を含む)で使われた:
| The ESCRT protein CHMP5 promotes T cell leukemia by enabling BRD4-p300-dependent transcription [ Nat Commun, 2025, 16(1):4133] | PubMed: 40319015 |
| Natural killer cell-derived granzyme B as a therapeutic target for alleviating graft injury during liver transplantation [ Acta Pharm Sin B, 2025, 15(10):5277-5293] | PubMed: 41132839 |
| Chidamide and cytarabine synergistically treat acute myeloid leukemia: inhibiting ribosome biogenesis via the MYC-RRP9 pathway [ Cell Death Dis, 2025, 16(1):601] | PubMed: 40781078 |
| The ADCY1-mediated cAMP signaling pathway mediates functional effects of montelukast treatment in brain organoids [ Cell Mol Life Sci, 2025, 82(1):224] | PubMed: 40471331 |
| The ADCY1-mediated cAMP signaling pathway mediates functional effects of montelukast treatment in brain organoids [ Cell Mol Life Sci, 2025, 82(1):224] | PubMed: 40471331 |
| Cysteine-reactive covalent chloro-N-acetamide ligands induce ferroptosis mediated cell death [ EMBO Rep, 2025, 10.1038/s44319-025-00593-4] | PubMed: 41102521 |
| Genome-wide CRISPR/Cas9 screen identifies AraC-daunorubicin-etoposide response modulators associated with outcomes in pediatric AML [ Blood Adv, 2025, 9(5):1078-1091] | PubMed: 39715471 |
| Mitochondrial dysfunction fuels drug resistance in adult T-cell acute lymphoblastic leukemia [ J Transl Med, 2025, 23(1):542] | PubMed: 40369632 |
| Establishment of a prognostic model based on ER stress-related cell death genes and proposing a novel combination therapy in acute myeloid leukemia [ J Transl Med, 2025, 23(1):566] | PubMed: 40399990 |
| Disulfidptosis in pediatric AML: a multi-omics approach to risk stratification and potential therapeutic strategy [ Cancer Cell Int, 2025, 25(1):199] | PubMed: 40457386 |
長期の保管のために-20°Cの下で製品を保ってください。
人間や獣医の診断であるか治療的な使用のためにでない。
各々の製品のための特定の保管と取扱い情報は、製品データシートの上で示されます。大部分のSelleck製品は、推薦された状況の下で安定です。製品は、推薦された保管温度と異なる温度で、時々出荷されます。長期の保管のために必要とされてそれと異なる温度で、多くの製品は、短期もので安定です。品質を維持するが、夜通しの積荷のために最も経済的な貯蔵状況を用いてあなたの送料を保存する状況の下に、製品が出荷されることを、我々は確実とします。製品の受領と同時に、製品データシートの上で貯蔵推薦に従ってください。
