|
受注:045-509-1970 |
技術サポート:tech@selleck.co.jp 平日9:00〜18:00 1営業日以内にご連絡を差し上げます |
化学情報
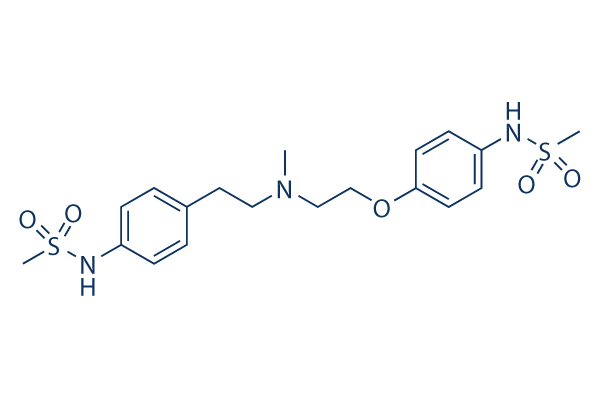
|
Synonyms | UK-68798 | Storage (From the date of receipt) |
3 years -20°C powder 1 years -80°C in solvent |
|||
| 化学式 | C19H27N3O5S2 |
||||||
| 分子量 | 441.56 | CAS No. | 115256-11-6 | ||||
| Solubility (25°C)* | 体外 | DMSO (warmed with 50ºC water bath) | 88 mg/mL (199.29 mM) | ||||
| Water | Insoluble | ||||||
| Ethanol | Insoluble | ||||||
| 体内 (毎回新しく調製した物を用意してください) |
|
||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. |
|||||||
溶剤液(一定の濃度)を調合する
生物活性
| 製品説明 | Dofetilide (UK-68798) is a selective potassium channel ((hERG)) blocker, used as a Class III antiarrhythmic drug. |
|---|---|
| in vitro | Dofetilide blocks HERG currents in excised macro patches of Xenopus oocytes. [1] This compound (1 μM) reduces the amplitude of IKr to 61% of control currents in guinea pig cardiomyocytes, as measured by 200-ms test pulses and analysis of the deactivating tail currents of IKr. [2] It increases apico-basal disparity of repolarization, due to a more marked increase of ERPs in the apex than in the base in the intact canine heart. [3] |
| in vivo | Dofetilide (100 mg/kg, i.v.) does not suppress automaticity arrhythmias induced by two-stage coronary ligation and epinephrine or the coronary ligation and reperfusion arrhythmias, but suppresses the reentry arrhythmia induced by PES in dogs with old myocardial infarction (MI). This compound also shows antiarrhythmic effect in some dogs with digitalis arrhythmia. It increases QT interval and shows negative chronotropic effect like that of other class III drugs, but is different in antiarrhythmic profiles from those of other class III agents such as D-sotalol, E-4031, and MS-551 in that it does not prevent the occurrence of ventricular fibrillation (VF) immediately after coronary reperfusion and has some antiarrhythmic effects on digitalis arrhythmia. [4] This chemical causes increased resorptions and the same stage-dependent malformations in Sprague-Dawley rats. [5] |
プロトコル(参考用のみ)
参考
|
Selleckの高級品が、幾つかの出版された研究調査結果(以下を含む)で使われた:
| R406 and its structural analogs reduce SNCA/α-synuclein levels via autophagic degradation [ Autophagy, 2025, 1-17.] | PubMed: 40143425 |
| Identification of amitriptyline HCl, flavin adenine dinucleotide, azacitidine and calcitriol as repurposing drugs for influenza A H5N1 virus-induced lung injury. [ PLoS Pathog, 2020, 16;16(3):e1008341] | PubMed: 32176725 |
| Human ileal organoid model recapitulates clinical incidence of diarrhea associated with small molecule drugs [ Toxicol In Vitro, 2020, 104928] | PubMed: 32622998 |
| Clinical Trial in a Dish: Personalized Stem Cell-Derived Cardiomyocyte Assay Compared With Clinical Trial Results for Two QT-Prolonging Drugs. [ Clin Transl Sci, 2019, 12(6):687-697] | PubMed: 31328865 |
| Development and validation of a UPLC-MS/MS analytical method for dofetilide in mouse plasma and urine, and its application to pharmacokinetic study. [ J Pharm Biomed Anal, 2019, 172:183-188] | PubMed: 31055183 |
| Increased Late Sodium Current Contributes to the Electrophysiological Effects of Chronic, but Not Acute, Dofetilide Administration. [ Circ Arrhythm Electrophysiol, 2016, 9(4):e003655] | PubMed: 27071826 |
| Structural and functional screening in human induced-pluripotent stem cell-derived cardiomyocytes accurately identifies cardiotoxicity of multiple drug types. [Doherty KR, et al. Toxicol Appl Pharmacol, 2015, 285(1):51-60] | PubMed: 25841593 |
長期の保管のために-20°Cの下で製品を保ってください。
人間や獣医の診断であるか治療的な使用のためにでない。
各々の製品のための特定の保管と取扱い情報は、製品データシートの上で示されます。大部分のSelleck製品は、推薦された状況の下で安定です。製品は、推薦された保管温度と異なる温度で、時々出荷されます。長期の保管のために必要とされてそれと異なる温度で、多くの製品は、短期もので安定です。品質を維持するが、夜通しの積荷のために最も経済的な貯蔵状況を用いてあなたの送料を保存する状況の下に、製品が出荷されることを、我々は確実とします。製品の受領と同時に、製品データシートの上で貯蔵推薦に従ってください。
