|
受注:045-509-1970 |
技術サポート:[email protected] 平日9:00〜18:00 1営業日以内にご連絡を差し上げます |
化学情報
|
Synonyms | INCB018424 | Storage (From the date of receipt) |
3 years -20°C powder 1 years -80°C in solvent |
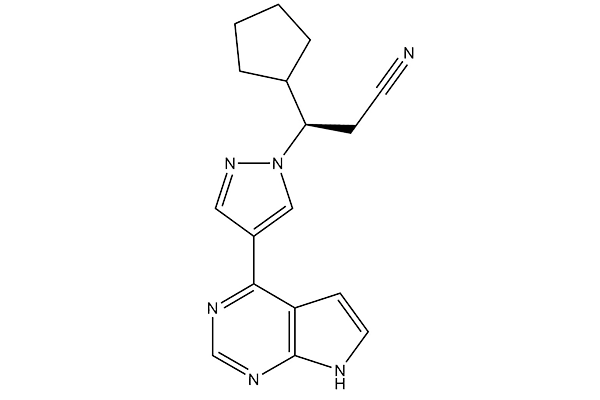
| 化学式 | C17H18N6 |
|||
| 分子量 | 306.37 | CAS No. | 941678-49-5 | |
| Solubility (25°C)* | 体外 | DMSO | 61 mg/mL (199.1 mM) | |
| Ethanol | 61 mg/mL (199.1 mM) | |||
| Water | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. |
||||
溶剤液(一定の濃度)を調合する
生物活性
| 製品説明 | ルクソリチニブ (Ruxolitinib (INCB018424)) は、最初に臨床試験が行われた強力で選択的なJAK1/2 阻害剤であり、IC50 はそれぞれ 3.3 nM/ 2.8 nM、JAK1/2 に対して JAK3 の 130 倍の選択性を示します。ルクソリチニブはマイトファジー (mitophagy) によって抗腫瘍作用を示します。また、オートファジー (autophagy) を誘導し、アポトーシス (apoptosis) を促進します。 |
|---|---|
| in vitro | INCB018424 potently and selectively inhibits JAK2V617F-mediated signaling and proliferation in Ba/F3 cells and HEL cells. INCB018424 markedly increases apoptosis in a dose dependent manner in Ba/F3 cells. INCB018424 (64 nM) results in a doubling of cells with depolarized mitochondria in Ba/F3 cells. INCB018424 inhibits proliferating of erythroid progenitors from normal donors and polycythemia vera patients with IC50 of 407 nM and 223 nM, respectively. INCB018424 demonstrates remarkable potency against erythroid colony formation with IC50 of 67nM. [1] |
| in vivo | INCB018424 (180 mg/kg, orally, twice a day) results in survive rate of greater than 90% by day 22 in a JAK2V617F-driven mouse model. INCB018424 (180 mg/kg, orally, twice a day) markedly reduces splenomegaly and circulating levels of inflammatory cytokines, and preferentially eliminated neoplastic cells, resulting in significantly prolonged survival without myelosuppressive or immunosuppressive effects in a JAK2V617F-driven mouse model. [1] The primary end point is reached in 41.9% of patients in the Ruxolitinib group as compared with 0.7% in the placebo group in the double-blind trial of myelofibrosis. Ruxolitinib results in maintaining of reduction in spleen volume and improvement of 50% or more in the total symptom score. [2] A total of 28% of the patients in the Ruxolitinib (15 mg twice daily) group has at least a 35% reduction in spleen volume at week 48 in patients with myelofibrosis, as compared with 0% in the group receiving the best available therapy. The mean palpable spleen length has decreased by 56% with Ruxolitinib but has increased by 4% with the best available therapy at week 48. Patients in the ruxolitinib group has an improvement in overall quality-of-life measures and a reduction in symptoms associated with myelofibrosis. [3] |
プロトコル(参考用のみ)
| キナーゼアッセイ | Binding assay | |
|---|---|---|
| Recombinant proteins are expressed using Sf21 cells and baculovirus vectors and purified with affinity chromatography. JAK kinase assays use a homogeneous time-resolved fluorescence assay with the peptide substrate (-EQEDEPEGDYFEWLE). Each enzyme reaction is carried out with Ruxolitinib or control, JAK enzyme, 500 nM peptide, adenosine triphosphate (ATP; 1mM), and 2% dimethyl sulfoxide (DMSO) for 1 hour. The 50% inhibitory concentration (IC50) is calculated as INCB018424 concentration required for inhibition of 50% of the fluorescent signal. | ||
| 細胞アッセイ | 細胞株 | Ba/F3 and HEL cells |
| 濃度 | 3 μM | |
| 反応時間 | 48 hours | |
| 実験の流れ | Cells are seeded at 2 × 103/well of white bottom 96-well plates, treated with INCB018424 from DMSO stocks (0.2% final DMSO concentration), and incubated for 48 hours at 37 ℃ with 5% CO2. Viability is measured by cellular ATP determination using the Cell-Titer Glo luciferase reagent or viable cell counting. Values are transformed to percent inhibition relative to vehicle control, and IC50 curves are fitted according to nonlinear regression analysis of the data using PRISM GraphPad. | |
| 動物実験 | 動物モデル | JAK2V617F-driven mouse model |
| 投薬量 | 180 mg/kg | |
| 投与方法 | Oral gavage | |
参考
|
カスタマーフィードバック
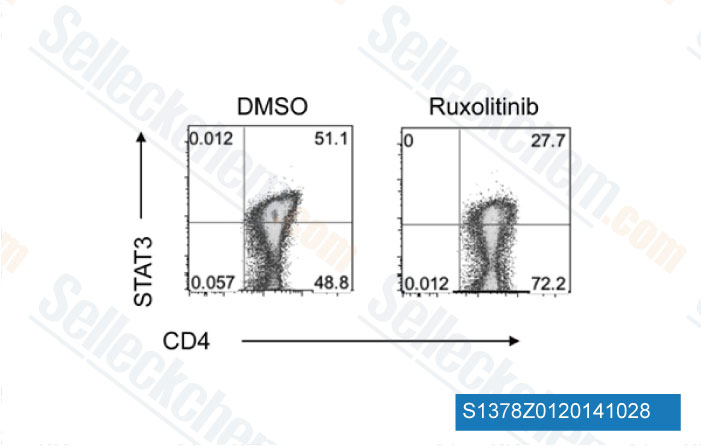
-
Data from [Data independently produced by Blood, 2014, 123(24), 3832-42]
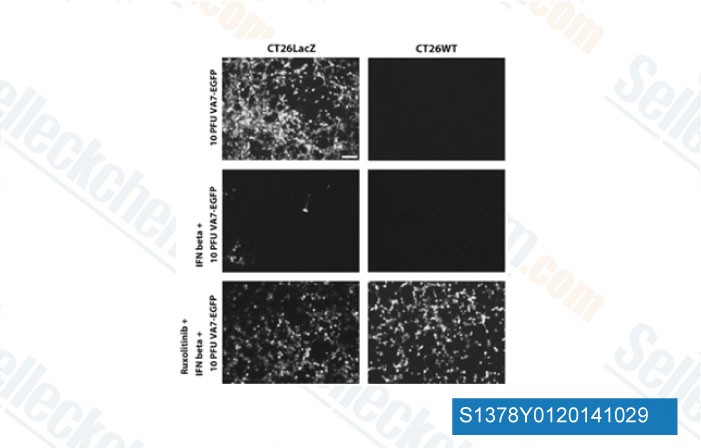
-
Data from [Data independently produced by Gene Ther, 2014, 10.1038/gt.2014.83]
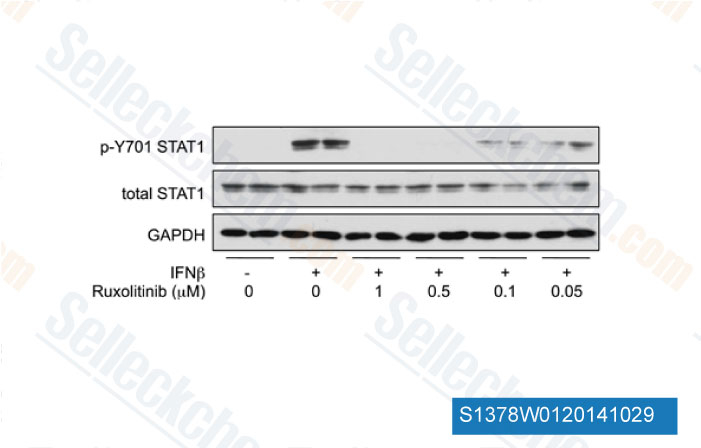
-
Data from [Data independently produced by J Immunol, 2012, 189(6), 2784-92]
Selleckの高級品が、幾つかの出版された研究調査結果(以下を含む)で使われた:
| Combined KRAS-MAPK pathway inhibitors and HER2-directed drug conjugate is efficacious in pancreatic cancer [ Nat Commun, 2024, 15(1):2503] | PubMed: 38509064 |
| Neuroinflammatory disease signatures in SPG11-related hereditary spastic paraplegia patients [ Acta Neuropathol, 2024, 147(1):28] | PubMed: 38305941 |
| Tumor cell senescence-induced macrophage CD73 expression is a critical metabolic immune checkpoint in the aging tumor microenvironment [ Theranostics, 2024, 14(3):1224-1240] | PubMed: 38323313 |
| XRN1 deletion induces PKR-dependent cell lethality in interferon-activated cancer cells [ Cell Rep, 2024, 43(2):113600.] | PubMed: 38261514 |
| Activation of cGAS-STING suppresses coxsackievirus replication via interferon-dependent signaling [ Antiviral Res, 2024, 222:105811] | PubMed: 38242503 |
| CD169+ classical monocyte as an important participant in Graves' ophthalmopathy through CXCL12-CXCR4 axis [ iScience, 2024, 27(3):109213] | PubMed: 38439953 |
| Functional analysis of a first hindlimb positioning enhancer via Gdf11 expression [ Front Cell Dev Biol, 2024, 12:1302141] | PubMed: 38559809 |
| Ovarian cancer ascites confers platinum chemoresistance to ovarian cancer cells [ Transl Oncol, 2024, 44:101939] | PubMed: 38489872 |
| A panel of janus kinase inhibitors identified with anti-inflammatory effects protect mice from lethal influenza virus infection [ Antimicrob Agents Chemother, 2024, 68(4):e0135023.] | PubMed: 38470034 |
| Calreticulin and JAK2V617F driver mutations induce distinct mitotic defects in myeloproliferative neoplasms [ Sci Rep, 2024, 14(1):2810] | PubMed: 38308077 |
長期の保管のために-20°Cの下で製品を保ってください。
人間や獣医の診断であるか治療的な使用のためにでない。
各々の製品のための特定の保管と取扱い情報は、製品データシートの上で示されます。大部分のSelleck製品は、推薦された状況の下で安定です。製品は、推薦された保管温度と異なる温度で、時々出荷されます。長期の保管のために必要とされてそれと異なる温度で、多くの製品は、短期もので安定です。品質を維持するが、夜通しの積荷のために最も経済的な貯蔵状況を用いてあなたの送料を保存する状況の下に、製品が出荷されることを、我々は確実とします。製品の受領と同時に、製品データシートの上で貯蔵推薦に従ってください。
