|
受注:045-509-1970 |
技術サポート:tech@selleck.co.jp 平日9:00〜18:00 1営業日以内にご連絡を差し上げます |
化学情報
|
Synonyms | N/A | Storage (From the date of receipt) |
3 years -20°C powder 1 years -80°C in solvent |
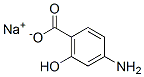
| 化学式 | C7H7NO3.2H2O.Na |
|||
| 分子量 | 211.15 | CAS No. | 6018-19-5 | |
| Solubility (25°C)* | 体外 | DMSO | 42 mg/mL (198.91 mM) | |
| Water | 42 mg/mL (198.91 mM) | |||
| Ethanol | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. |
||||
溶剤液(一定の濃度)を調合する
生物活性
| 製品説明 | Sodium 4-Aminosalicylate is an antibiotic used to treat tuberculosis via NF-κB inhibition and free radical scavenging. |
|---|---|
| in vitro | 4-Aminosalicylate reacts promptly with DPPH, suggesting a potent radical scavenger activity. 4-Aminosalicylate exhibits peroxyl radical scavenging activity generated by the water-soluble 2,2'-azobis-(2-amidinopropane hydrochloride) azoinitiator of peroxyl radicals, as evidenced by the inhibition of cis-parinaric acid fluorescence decay or oxygen consumption. 4-Aminosalicylate rapidly scavenges peroxyl radicals in the aqueous phase, producing a concentration-dependent inhibition period similar to Trolox or cysteine, suggesting an antioxidant activity of chain-breaking type. [1] [14C]4-Aminosalicylate transforms to a number of metabolites, among which we have characterized salicylate and gentisate, in activated mononuclear cells and activated granulocytes. 4-Aminosalicylate (0.65 mM) diminishes the lethal effect on cultured Chinese hamster ovary cells of adding either superoxide radical or hydrogen peroxide. [2] Aminosalicylate (25 mM) stimulates phospholipase D in a time- and concentration-dependent manner via a pathway involving inositol 1,4,5-trisphosphate generation, calcium fluxes, and Gi/Go in cultured mouse peritoneal macrophages. 4-aminosalicylate (20 mM) increases the levels of inositol 1,4,5-trisphosphate by 260% after treatment of macrophages. 4-aminosalicylate (5 mM) potentiates the activation of PLD by protein kinase C in cultured mouse peritoneal macrophages. [3] 4-aminosalicylate (0.1 mM) decreases the LTB4 synthesis in a dose-related fashion in isolated colonic mucosal cells, thus diminishes the LTB4/PGE2 ratio. [4] 4-aminosalicylate (0.1 mg/mL) is preferentially transported in the basolateral (BL) to apical (AP) direction, and the N-acetyl metabolite appeared only in the AP compartment. [5] |
| in vivo | 4-aminosalicylate (7.5 mg/mL, regional perfusions) results in the appearance of N-acetyl-5-aminosalicylic acid in the intestinal lumen in the anesthetized rat. [5] |
| 特徴 | 4-Aminosalicylate is considered to be the active moiety of sulfasalazine. |
プロトコル(参考用のみ)
参考
|
カスタマーフィードバック
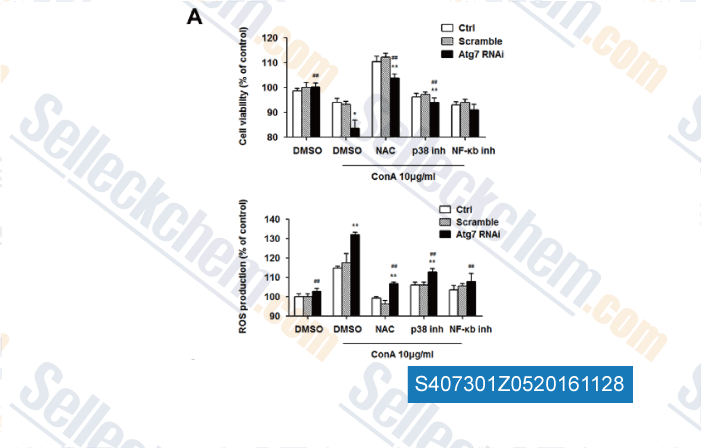
-
Data from [Data independently produced by , , PLoS One, 2016, 11(3):e0149754.]
Selleckの高級品が、幾つかの出版された研究調査結果(以下を含む)で使われた:
| Silibinin inhibits in vitro ketosis by regulating HMGCS2 and NF-kB: elucidation of signaling molecule relationship under ketotic conditions [ In Vitro Cell Dev Biol Anim, 2019, 55(5):368-375] | PubMed: 31025252 |
| Atg7 Knockdown Augments Concanavalin A-Induced Acute Hepatitis through an ROS-Mediated p38/MAPK Pathway. [Zhuang Y, et al. PLoS One, 2016, 11(3):e0149754] | PubMed: 26939081 |
長期の保管のために-20°Cの下で製品を保ってください。
人間や獣医の診断であるか治療的な使用のためにでない。
各々の製品のための特定の保管と取扱い情報は、製品データシートの上で示されます。大部分のSelleck製品は、推薦された状況の下で安定です。製品は、推薦された保管温度と異なる温度で、時々出荷されます。長期の保管のために必要とされてそれと異なる温度で、多くの製品は、短期もので安定です。品質を維持するが、夜通しの積荷のために最も経済的な貯蔵状況を用いてあなたの送料を保存する状況の下に、製品が出荷されることを、我々は確実とします。製品の受領と同時に、製品データシートの上で貯蔵推薦に従ってください。
