|
受注:045-509-1970 |
技術サポート:[email protected] 平日9:00〜18:00 1営業日以内にご連絡を差し上げます |
化学情報
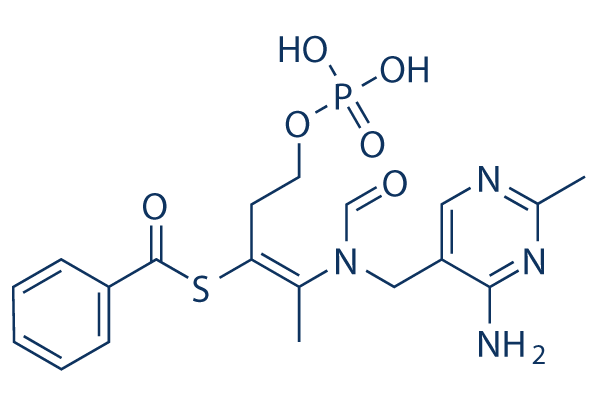
|
Synonyms | S-Benzoylthiamine O-monophosphate, Benzoylthiamine monophosphate | Storage (From the date of receipt) |
3 years -20°C powder 1 years -80°C in solvent |
| 化学式 | C19H23N4O6PS |
|||
| 分子量 | 466.45 | CAS No. | 22457-89-2 | |
| Solubility (25°C)* | 体外 | 5%TFA | 6 mg/mL (12.86 mM) | |
| DMSO | 0.01 mg/mL (0.02 mM) | |||
| Water | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. |
||||
溶剤液(一定の濃度)を調合する
生物活性
| 製品説明 | Benfotiamine (S-Benzoylthiamine O-monophosphate) is a synthetic S-acyl derivative of thiamine (vitamin B1) and has been investigated for the treatment and prevention of Diabetic Nephropathy and Diabetes Mellitus, Type 2. Benfotiamine suppresses oxidative stress-induced NF-κB activation and prevents the bacterial endotoxin-induced inflammation. |
|---|---|
| in vitro | Benfotiamine improves the expression of endothelial cell markers in EPCs, restores eNOS levels, and recovers the ability of EPCs to participate in angiogenic processes. It is able to dampen glucose toxicity effects on endothelial progenitors[1]. Benfotiamine possesses antitumor activity against leukemia cells. In a panel of nine myeloid leukemia cell lines benfotiamine impairs the viability of HL-60, NB4, K562 and KG1 cells and also inhibits the growing of primary leukemic blasts. The antitumor activity of benfotiamine is not mediated by apoptosis, necrosis or autophagy, but rather occurs though paraptosis cell death induction. Benfotiamine inhibits the activity of constitutively active ERK1/2 and concomitantly increases the phosphorylation of JNK1/2 kinase in leukemic cells. In addition, benfotiamine induces the down regulation of the cell cycle regulator CDK3 which results in G1 cell cycle arrest in the sensitive leukemic cells[2]. |
| in vivo | Benfotiamine might exert vascular and renal benefits by modulating mechanisms independent or downstream of ROS formation. Benfotiamine aids the post-ischaemic healing of diabetic animals via PKB/Akt-mediated potentiation of angiogenesis and inhibition of apoptosis[3]. |
プロトコル(参考用のみ)
| 細胞アッセイ | 細胞株 | leukemia cells (HL60, AML 1, NB4 cells) |
|---|---|---|
| 濃度 | 50 μM | |
| 反応時間 | 24, 48, 72, 96 h | |
| 実験の流れ | Cell viability is assessed using a colorimetric MTT metabolic activity assay. |
|
| 動物実験 | 動物モデル | diabetic animal model induced by STZ (male CD1 mice) |
| 投薬量 | 80 mg/kg | |
| 投与方法 | oral administration |
参考
Selleckの高級品が、幾つかの出版された研究調査結果(以下を含む)で使われた:
| Repurposing the natural compounds as potential therapeutic agents for COVID-19 based on the molecular docking study of the main protease and the receptor-binding domain of spike protein [ J Mol Model, 2022, 28(6):153] | PubMed: 35578055 |
長期の保管のために-20°Cの下で製品を保ってください。
人間や獣医の診断であるか治療的な使用のためにでない。
各々の製品のための特定の保管と取扱い情報は、製品データシートの上で示されます。大部分のSelleck製品は、推薦された状況の下で安定です。製品は、推薦された保管温度と異なる温度で、時々出荷されます。長期の保管のために必要とされてそれと異なる温度で、多くの製品は、短期もので安定です。品質を維持するが、夜通しの積荷のために最も経済的な貯蔵状況を用いてあなたの送料を保存する状況の下に、製品が出荷されることを、我々は確実とします。製品の受領と同時に、製品データシートの上で貯蔵推薦に従ってください。
