|
受注:045-509-1970 |
技術サポート:tech@selleck.co.jp 平日9:00〜18:00 1営業日以内にご連絡を差し上げます |
化学情報
|
Synonyms | Bay h 4502,(±)-bifonazole | Storage (From the date of receipt) |
3 years -20°C powder 1 years -80°C in solvent |
|||
| 化学式 | C22H18N2 |
||||||
| 分子量 | 310.39 | CAS No. | 60628-96-8 | ||||
| Solubility (25°C)* | 体外 | DMSO | 62 mg/mL (199.74 mM) | ||||
| Ethanol | 20 mg/mL (64.43 mM) | ||||||
| Water | Insoluble | ||||||
| 体内 (毎回新しく調製した物を用意してください) |
|
||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. |
|||||||
溶剤液(一定の濃度)を調合する
生物活性
| 製品説明 | Bifonazole (Bay h 4502,(±)-bifonazole)は、置換イミダゾール系のFungal剤です。 |
|---|---|
| in vitro | Bifonazole, a broad spectrum antifungal agent, inhibits monooxygenase activity and induces a type II binding spectrum in 2B4dH(H226Y), a modified enzyme previously crystallized in the presence of 4-(4-chlorophenyl)imidazole (CPI). This compound (40 mM) releases Ca2+ from the store sensitive to 1 mM thapsigargin, an endopolasmic reticulum Ca2+ pump inhibitor. It per se induces capacitative Ca2+ entry while reduces 1 mM thapsigargin-induced capacitative Ca2+ entry. This chemical is calmodulin antagonists which most effectively reduce glycolysis and ATP level in B16 melanoma cells. It acts through allosteric regulation and detachment of glycolytic enzymes from cytoskeleton. This agent blocks PGE2 formation induced by 2 μm and 4 μm arachidonic acid clearly better than at 6 or 10 μm of the agonist. It shows the same characteristics in MC3T3-E1 and UMR-106 cells stimulated by ionomycin or various concentrations of arachidonic acid. |
| in vivo | Bifonazole, but not clotrimazole, exhibits the characteristics of a peroxisome proliferator including hepatomegaly (increase in liver:body weight ratio), up to a 4-fold induction of lauric acid omega-hydroxylase activity and an 8-fold induction of palmitoyl-CoA oxidation by rat liver peroxisomes. This compound also induces P402B1/2B2, P4503A and P4501A1, but not P4502E1. |
プロトコル(参考用のみ)
参考
|
カスタマーフィードバック
-
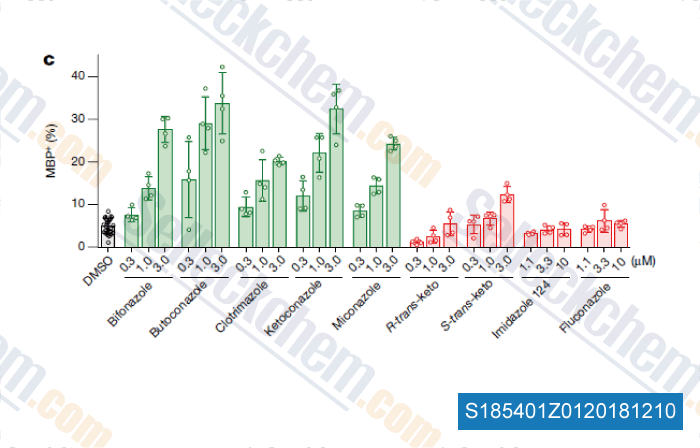
Data from [Data independently produced by , , Nature, 2018, 560(7718):372-376]
Selleckの高級品が、幾つかの出版された研究調査結果(以下を含む)で使われた:
| Accumulation of 8,9-unsaturated sterols drives oligodendrocyte formation and remyelination [Hubler Z, et al. Nature, 2018, 560(7718):372-376] | PubMed: 30046109 |
長期の保管のために-20°Cの下で製品を保ってください。
人間や獣医の診断であるか治療的な使用のためにでない。
各々の製品のための特定の保管と取扱い情報は、製品データシートの上で示されます。大部分のSelleck製品は、推薦された状況の下で安定です。製品は、推薦された保管温度と異なる温度で、時々出荷されます。長期の保管のために必要とされてそれと異なる温度で、多くの製品は、短期もので安定です。品質を維持するが、夜通しの積荷のために最も経済的な貯蔵状況を用いてあなたの送料を保存する状況の下に、製品が出荷されることを、我々は確実とします。製品の受領と同時に、製品データシートの上で貯蔵推薦に従ってください。
