|
受注:045-509-1970 |
技術サポート:tech@selleck.co.jp 平日9:00〜18:00 1営業日以内にご連絡を差し上げます |
化学情報
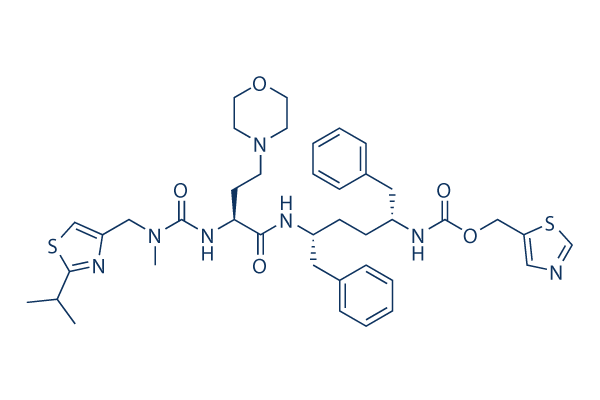
|
Synonyms | GS-9350 | Storage (From the date of receipt) |
3 years -20°C powder 1 years -80°C in solvent |
|||||||
| 化学式 | C40H53N7O5S2 |
||||||||||
| 分子量 | 776.02 | CAS No. | 1004316-88-4 | ||||||||
| Solubility (25°C)* | 体外 | DMSO | 100 mg/mL (128.86 mM) | ||||||||
| Ethanol | 100 mg/mL (128.86 mM) | ||||||||||
| Water | Insoluble | ||||||||||
| 体内 (毎回新しく調製した物を用意してください) |
|
||||||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. |
|||||||||||
溶剤液(一定の濃度)を調合する
生物活性
| 製品説明 | Cobicistat is a potent and selective inhibitor of CYP3A with IC50 of 30-285 nM. |
|---|---|
| in vitro | Cobicistat (GS-9350) is a potent, and selective inhibitor of human cytochrome P450 3A (CYP3A) enzymes as a pharmacoenhancer. GS-9350 inhibits CYP3A with IC50 spectrum from 30 nM to 285 nM. In contrast to ritonavir, GS-9350 is devoid of anti-HIV activity, with IC50 of > 30μM against HIV-1 protease and EC50 of > 30μM in MT-2 HIV infection assay, and is thus more suitable for use in boosting anti-HIV drugs without risking selection of potential drug-resistant HIV variants. GS-9350 shows reduced liability for drug interactions and may have potential improvements in tolerability over ritonavir. [1] |
プロトコル(参考用のみ)
| キナーゼアッセイ | Cytochrome P450 Inhibition | |
|---|---|---|
| Inhibition of human cytochrome P450 activities is determined in duplicate in pooled human hepatic microsomal fractions following current scientific and regulatory guidelines. Reaction conditions are linear with respect to incubation time and hepatic microsomal protein concentration. Substrates are present at concentrations equal to or less than their respective Km values determined under the same reaction conditions. Metabolite and/or substrate concentrations are determined using specific, internal standard controlled HPLC MS/MS assays. For reactions monitoring metabolite formation there is less than 20% consumption of substrate during the reaction. Unless otherwise noted microsomal fraction, diluted in potassium phosphate buffer, is preincubated with substrate and inhibitor for 5 min at 37 ℃ and the reaction initiated by the addition of an NADPH generating system followed by further incubation at 37 ℃ with shaking. Enzyme-selective positive control inhibitors are tested in parallel. At appropriate times aliquots of the mixture are removed and the reaction terminated by addition to a mixture of methanol and acetonitrile containing the respective internal standard. After centrifugation aliquots of the supernatant are subjected to HPLC-MS/MS analysis. | ||
| 細胞アッセイ | 細胞株 | MT-2 |
| 濃度 | ||
| 反応時間 | 5 days | |
| 実験の流れ | Five-fold serial dilutions of the tested compounds are prepared in triplicate in 96-well plates. MT-2 cells are added to plates at a density of 20,000/well in a final assay volume of 200μL. After a 5-day incubation at 37 ℃, the cytotoxic effect is determined using a cell viability assay. One hundredμL media is removed from each well and replaced with 100μL of phosphate-buffered saline containing 1.7 mg/mL XTT and 5μg/mL PMS. Following 1-hour incubation at 37 ℃, 20μL of 2% Triton X- 100 is added to each well and absorbance is read at 450 nm with a background subtraction at 650 nm. The data are plotted as cell viability vs. drug concentration. Cell viability is expressed as a percentage of the signal from untreated samples (0% cytotoxicity) after the subtraction of signal from samples treated with 10μM of Podophyllotoxin (100% cytotoxicity). The CC50 value is calculated from the inhibition plots as the concentration of drug which inhibited cell proliferation by 50%. | |
参考
|
カスタマーフィードバック
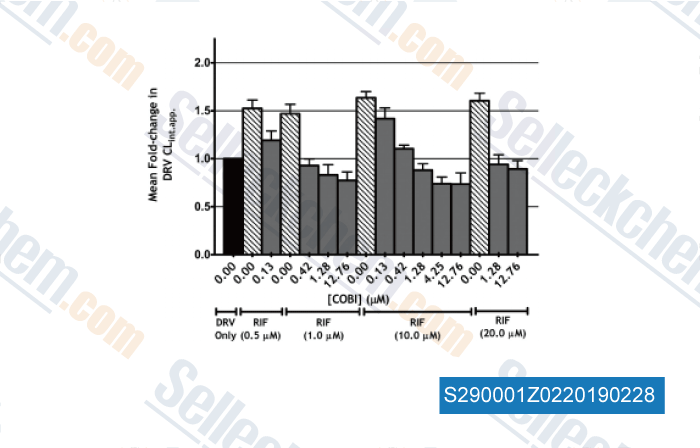
-
Data from [Data independently produced by , , Antimicrob Agents Chem, 2017, doi:10.1128/AAC.01776-16]
Selleckの高級品が、幾つかの出版された研究調査結果(以下を含む)で使われた:
| Patient-derived rhabdomyosarcoma cells recapitulate the genetic and transcriptomic landscapes of primary tumors [ iScience, 2024, 27(10):110862] | PubMed: 39319271 |
| Establishment and Characterization of NCC-PMP1-C1: A Novel Patient-Derived Cell Line of Metastatic Pseudomyxoma Peritonei [ J Pers Med, 2022, 12(2)258] | PubMed: 35207746 |
| Establishment and characterization of NCC-UPS4-C1: a novel cell line of undifferentiated pleomorphic sarcoma from a patient with Li-Fraumeni syndrome [ Hum Cell, 2022, 10.1007/s13577-022-00671-y] | PubMed: 35118583 |
| Establishment and characterization of NCC-MFS4-C1: a novel patient-derived cell line of myxofibrosarcoma [ Hum Cell, 2021, 34(6):1911-1918] | PubMed: 34383271 |
| Establishment and characterization of the NCC-GCTB4-C1 cell line: a novel patient-derived cell line from giant cell tumor of bone [ Hum Cell, 2021, 10.1007/s13577-021-00639-4] | PubMed: 34731453 |
| Establishment and characterization of novel patient-derived cell lines from giant cell tumor of bone [ Hum Cell, 2021, 10.1007/s13577-021-00579-z] | PubMed: 34304386 |
| Establishment and characterization of patient-derived cancer models of malignant peripheral nerve sheath tumors. [ Cancer Cell Int, 2020, 19;20:58] | PubMed: 32099531 |
| Establishment and characterization of NCC-MFS2-C1: a novel patient-derived cancer cell line of myxofibrosarcoma [ Hum Cell, 2020, 10.1007/s13577-020-00420-z] | PubMed: 32870449 |
| Establishment and characterization of a novel cell line, NCC-TGCT1-C1, derived from a patient with tenosynovial giant cell tumor [ Hum Cell, 2020, 10.1007/s13577-020-00425-8] | PubMed: 32886306 |
| Human Platelet Dysregulation by Antiretroviral Drugs and Cigarette Smokein vitro [ ProQuest, 2019, 13863770] | PubMed: None |
長期の保管のために-20°Cの下で製品を保ってください。
人間や獣医の診断であるか治療的な使用のためにでない。
各々の製品のための特定の保管と取扱い情報は、製品データシートの上で示されます。大部分のSelleck製品は、推薦された状況の下で安定です。製品は、推薦された保管温度と異なる温度で、時々出荷されます。長期の保管のために必要とされてそれと異なる温度で、多くの製品は、短期もので安定です。品質を維持するが、夜通しの積荷のために最も経済的な貯蔵状況を用いてあなたの送料を保存する状況の下に、製品が出荷されることを、我々は確実とします。製品の受領と同時に、製品データシートの上で貯蔵推薦に従ってください。
