|
受注:045-509-1970 |
技術サポート:tech@selleck.co.jp 平日9:00〜18:00 1営業日以内にご連絡を差し上げます |
化学情報
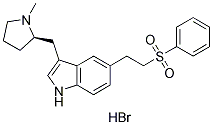
|
Synonyms | UK-116044 | Storage (From the date of receipt) |
3 years -20°C powder 1 years -80°C in solvent |
|||||||||||
| 化学式 | C22H26N2O2S.HBr |
||||||||||||||
| 分子量 | 463.43 | CAS No. | 177834-92-3 | ||||||||||||
| Solubility (25°C)* | 体外 | DMSO | 93 mg/mL (200.67 mM) | ||||||||||||
| Water | Insoluble | ||||||||||||||
| Ethanol | Insoluble | ||||||||||||||
| 体内 (毎回新しく調製した物を用意してください) |
|
||||||||||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. |
|||||||||||||||
溶剤液(一定の濃度)を調合する
生物活性
| 製品説明 | Eletriptan (UK-116044) is a selective 5-HT1B and 5-HT1D receptor agonist with Ki of 0.92 nM and 3.14 nM, respectively. |
|---|---|
| in vitro | [3H]Eletriptan has a total number of binding sites (Bmax) of 2478 fmol/mg and 1576 fmol/mg for 5-HT1B and 5-HT1D, respectively. [3H]Eletriptan has a significantly faster association rate (K(on) 0.249/min/nM) than [3H]sumatriptan (K(on) 0.024/min/nM) and a significantly slower off-rate (K(off) 0.027/min compared to 0.037/min for [3H]sumatriptan). [1] Eletriptan induces concentration-dependent contractions of meningeal artery, coronary artery, and saphenous vein. The potency of Eletriptan is higher in meningeal artery than in coronary artery (86-fold) or saphenous vein (66-fold). The predicted contraction by Eletriptan (40 mg and 80 mg) and sumatriptan (100 mg) at free C(max) observed in clinical trials is similar in meningeal artery. [2] |
| in vivo | Eletriptan (<1000 mg/kg, i.v.) produces a dose-dependent reduction of carotid arterial blood flow in the anaesthetised dog. Eletriptan reduces coronary artery diameter with ED50 value of 63 mg/kg in the anaesthetised dog. Eletriptan (<300 mg/kg, i.v.) administered prior to electrical stimulation of the trigeminal ganglion produces a dose-related and complete inhibition of plasma protein extravasation in the dura mater rats. Eletriptan (100 mg/kg, i.v.) produces a complete inhibition of plasma protein extravasation in rat dura mater. [3] Headache response rates are 24% for placebo; 54% for Eletriptan (20 mg);65% for Eletriptan (40 mg);and 77% for Eletriptan (80 mg) at the primary endpoint (2 hours after dosing) in patients with migraine. Headache-free rates at 2 hours are 6% for placebo, 29% for Eletriptan (40 mg) and 37% for Eletriptan (80 mg) at the primary endpoint (2 hours after dosing) in patients with migraine. Eletriptan is well tolerated, and the majority of adverse events are mild or moderate in intensity and transient in patients with migraine. [4] Iontophoretic ejection (50 nA) of Eletriptan suppresses the response in 75% of cells and causes an average suppression of cell firing of 42% in cats. [5] |
プロトコル(参考用のみ)
| キナーゼアッセイ | Binding assay | |
|---|---|---|
| Binding studies are performed in a total assay volume of 500 mL containing final protein concentrations of 50–150 mg/mL. Time to equilibrium is determined by incubating 50 mL of [3H]Eletriptan (85 Ci/mmol) with 400 mL of either 5-HT1B or 5-HT1D membrane homogenate for time points ranging from 1 to 60 min. These experiments are performed at either 4℃ or 22℃ using a KD, or lower, concentration of the appropriate radioligand, as determined from saturation assays. Total and non-specific binding are determined in duplicate at each time point. Non-specific binding is defined by 10 μM 5-HT. Following an initial incubation period of 30 min at either 4℃ or 22℃, dissociation is initiated by addition of 10 mM 5-HT, an excess concentration which over time would effectively displace all radioligand from the receptors. Total and non-specific binding are determined in duplicate at time points ranging from 1min–90 min. | ||
参考
|
長期の保管のために-20°Cの下で製品を保ってください。
人間や獣医の診断であるか治療的な使用のためにでない。
各々の製品のための特定の保管と取扱い情報は、製品データシートの上で示されます。大部分のSelleck製品は、推薦された状況の下で安定です。製品は、推薦された保管温度と異なる温度で、時々出荷されます。長期の保管のために必要とされてそれと異なる温度で、多くの製品は、短期もので安定です。品質を維持するが、夜通しの積荷のために最も経済的な貯蔵状況を用いてあなたの送料を保存する状況の下に、製品が出荷されることを、我々は確実とします。製品の受領と同時に、製品データシートの上で貯蔵推薦に従ってください。
