|
受注:045-509-1970 |
技術サポート:tech@selleck.co.jp 平日9:00〜18:00 1営業日以内にご連絡を差し上げます |
化学情報
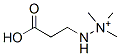
|
Synonyms | MET-88, Quaterin | Storage (From the date of receipt) |
3 years -20°C powder 1 years -80°C in solvent |
| 化学式 | C6H14N2O2 |
|||
| 分子量 | 147.19 | CAS No. | 76144-81-5 | |
| Solubility (25°C)* | 体外 | Water | 29 mg/mL (197.02 mM) | |
| Ethanol | 29 mg/mL (197.02 mM) | |||
| DMSO | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. |
||||
溶剤液(一定の濃度)を調合する
生物活性
| 製品説明 | Meldonium(MET-88、Quaterin)は、ガンマ-ブチロベタイン(GBB)ヒドロキシラーゼによるL-カルニチン生合成の阻害剤であり、腎臓のカルニチン再吸収の競合阻害剤でもあります。 |
|---|---|
| in vitro | Meldonium (40 μM) inhibits the reaction of γ-butyrobetaine hydroxylase with γ-butyrobetaine with Km and Vmax of 36.8 μM and 0.08 nmol/min/mg protein, respectively. |
| in vivo | Meldonium administered orally to rats for 10 days (150 mg/kg) elicits a reduction in myocardial free camitine and long-chain acyl carnitine content by 63.7 and 74.3%, respectively. This compound treatment (100 mg/kg, orally) subsequent administration of isoproterenol results in a reduction in free camitine concentration by 48.7% in comparison with the rats receiving isoproterenol. A prior administration of this chemical effectively protects the myocardium from isoproterenol-induced variations in the content of ATP and myocardial energy charge, as well as preventing a rise in creatine phosphokinase and lactic dehydrogenase activity. This compound (200 mg/kg) long-term treatment significantly increases the rate of insulin-stimulated glucose uptake by 35% and the expression of glucose transporter 4 (1.7-fold increase), hexokinase II (2.1-fold increase), insulin receptor proteins (2.5-fold increase) and carnitine palmitoyltransferases IA (2.2-fold increase) in mouse hearts. This chemical long-term treatment statistically significantly decreases fed state blood glucose from 6 to 5 mM. It reduces the azidothymidine-induced alterations in mouse brain tissue. This compound (50 mg/kg) normalizes the increase in caspase-3, cellular apoptosis susceptibility protein (CAS) and iNOS expression. It also normalizes the changes in cytochromec oxidase (COX) expression, reduces the expression of glial fibrillary acidic protein (GFAP) and cellular infiltration. This chemical displays protective effects in experimental model of type 2 diabetes in Goto-Kakizaki rats. It (200 mg/kg) treatment decreases both the fed- and fasted-state blood glucose. This compound strongly inhibits fructosamine accumulation and loss of pain sensitivity (by 75%) and also ameliorates the enhanced contractile responsiveness of Goto-Kakizaki rat aortic rings to phenylephrine. In addition, in this chemical-treated hearts, the necrosis zone following coronary occlusion is significantly decreased by 30%. |
プロトコル(参考用のみ)
| キナーゼアッセイ | γ-Butyrobetaine hydroxylase activity assay | |
|---|---|---|
| γ-Butyrobetaine hydroxylase activity is assessed by the amount of carnitine formed from γ-butyrobetaine. Camitine is assayed radiometrically with the aid of camitine acetyltransferase and 14C-acetyl-CoA. The 14C-acetyl carnitine is isolated by ion-exchange chromatography on Dowex 2 × 8 (Cl--form, 200-400 mesh) following enzymatic reactions. The incubation mixture (final volume 125 μL) consists of γ-butyrobetaine (75 μL, 6.65-26.6 μM), Meldonium solution (10 μL, 50-500 μM), 20 μL of ascorbate and α-ketoglutarate mixture (500 μL 37.5 mM sodium ascorbate, 375 μL 25 mM αketoglutarate, 125 μL 1 M potassium phosphate buffer, pH 6.7), 10 μL of γ-butyrobetaine hydroxylase and catalase mixture (150 μL of isolated γ-butyrobetaine hydroxylase solution, -77 mU, 50μL catalase solution -2 mg, 50 μL 100 mM potassium phosphate buffer, pH 6.7, 250 μL H2O), ferrous ammonium sulphate (10μL, 6.25 mM). Following incubation for 30 min at 30 ℃ the samples are supplemented with 25 μL 1.6 M KOH and kept for 20 min at 56 ℃. Then, 35 μL of the neutralising solution are added (1 mL 1 M HEPES acid, 400μL 0.5 M glutathione, 10 μL 100 mM EDTA, 250 μL 2 M H3/sub>PO4, water to 1.75 mL). Following neutralisation the sample should have pH 7.6. After adding 10 μL of l-14C-acetyl-CoA (2 nm, 15 nCi) and 5 μL of camitine acetyltransferase (21 μg protein) the mixture is incubated for 30 min at 30 ℃ and each sample is supplemented with 290 mg of dry Dowex resin 2 × 8 (Cl--form, 200-400 mesh) and 600 μL H2O. The samples are shaken for 10 min, spun down (2000 g × 5 min) and the supematant (0.4 mL), is mixed with Bray’s scintillation fluid (10 mL). Radioactivity is measured in an counter. | ||
参考
|
Selleckの高級品が、幾つかの出版された研究調査結果(以下を含む)で使われた:
| Dissection of immunotherapeutic predictive versus prognostic transcriptional programs identifies SLC22A5-centric carnitine metabolism-driven resistance to anti-PD-(L)1 treatment in non-small cell lung cancer [ Drug Resist Updat, 2025, 84:101313] | PubMed: 41045681 |
| αKG-mediated carnitine synthesis promotes homologous recombination via histone acetylation [ bioRxiv, 2024, 2024.02.06.578742] | PubMed: 38370789 |
| CRIP1 suppresses BBOX1-mediated carnitine metabolism to promote stemness in hepatocellular carcinoma [ EMBO J, 2022, 41(15):e110218] | PubMed: 35775648 |
| Identification of BBOX1 as a Therapeutic Target in Triple-Negative Breast Cancer [ Cancer Discov, 2020, CD-20-0288] | PubMed: 32690540 |
長期の保管のために-20°Cの下で製品を保ってください。
人間や獣医の診断であるか治療的な使用のためにでない。
各々の製品のための特定の保管と取扱い情報は、製品データシートの上で示されます。大部分のSelleck製品は、推薦された状況の下で安定です。製品は、推薦された保管温度と異なる温度で、時々出荷されます。長期の保管のために必要とされてそれと異なる温度で、多くの製品は、短期もので安定です。品質を維持するが、夜通しの積荷のために最も経済的な貯蔵状況を用いてあなたの送料を保存する状況の下に、製品が出荷されることを、我々は確実とします。製品の受領と同時に、製品データシートの上で貯蔵推薦に従ってください。
