|
受注:045-509-1970 |
技術サポート:tech@selleck.co.jp 平日9:00〜18:00 1営業日以内にご連絡を差し上げます |
化学情報
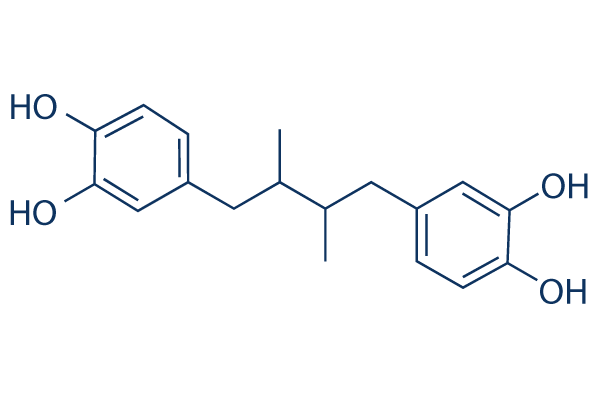
|
Synonyms | N/A | Storage (From the date of receipt) |
3 years -20°C powder (seal) | |||
| 化学式 | C18H22O4 |
||||||
| 分子量 | 302.36 | CAS No. | 500-38-9 | ||||
| Solubility (25°C)* | 体外 | DMSO | 60 mg/mL (198.43 mM) | ||||
| Ethanol | 60 mg/mL (198.43 mM) | ||||||
| Water | Insoluble | ||||||
| 体内 (毎回新しく調製した物を用意してください) |
|
||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. |
|||||||
溶剤液(一定の濃度)を調合する
生物活性
| 製品説明 | Nordihydroguaiaretic acid (NDGA)は、常緑の砂漠の低木であるLarrea tridentata (Sesse and Moc. ex DC) Coville(クレオソートブッシュ)の葉と小枝に見られるフェノール系抗酸化物質です。これはリポキシゲナーゼ(LOX)の既知の阻害剤であり、抗酸化作用とフリーラジカル捕捉作用を持っています。Nordihydroguaiaretic acid (NDGA)は、細胞毒性のあるインスリン様成長因子-I受容体(IGF-1R)/HER2阻害剤であり、アポトーシスを誘発します。Nordihydroguaiaretic acid (NDGA)はp300を阻害し、オートファジーを活性化します。Nordihydroguaiaretic acid (NDGA)は細胞をフェロトーシスから保護します。 |
|---|---|
| in vitro | NDGA has been proven to selectively inhibit arachidonic acid 5-lipoxygenase activity, which reduces leukotriene and prostaglandin synthesis, thus leading to a reduction of inflammatory pathways. This compound also has profound effects on the secretory pathway, reflected in its ability to block production of leukotriene B4, degranulation, phagocytosis, and the respiratory burst by exerting effects on the mitochondria and nonspecifically inhibiting NADPH oxidase and protein kinase C. It has also been shown to block protein transport from the endoplasmic reticulum (ER) to the Golgi complex, induce the redistribution of Golgi proteins into the ER and affect levels of intracellular calcium. Furthermore, this chemical has been shown to disrupt the actincytoskeleton and exert effects on cell adhesion and also to directly inhibit activationof two receptor tyrosine kinases (RTKs), the Insulin-like growth factor-1 receptor and the c-erbB2/HER2/neu receptor, that results in decreased cellular proliferation. It selectively inhibits platelet-derived growth factor (PDGF)-stimulated DNA synthesis in Swiss 3T3 cells, diploid murine cells and rat and human fibroblasts. This bioactive natural product is able to crosslink collagen. Its cross-linking may provide a viable approach to stabilizing collagenous materials for use in repair of ruptured, lacerated or surgically transected tendons, as well as other biomaterial constructs for surgical repair of musculoskeletal injuries and disease. |
| in vivo | Adding 0.1% NDGA to the drinking water of athymic mice bearing non-small cell lung cancer tumors significantly inhibits tumor growth compared with control mice. In addition, this compound has not only been shown to suppress breast cancer cell growth, it has a synergistic effect with retinoic acid on the inhibition of mammary tumor cell transformation and proliferation. Preliminary in vivo studies have revealed that this chemical suppresses tumor growth by inhibiting metabolic enzymes as well as RTK phosphorylation, which is overexpressed in certain cancer cells. It has also been proven to be a potent anti-ischemia-reperfusion injury agent in vitro and in animal models through different antioxidant pathways. It has been identified as a compound capable of inducing glutamate uptake and upregulation of expression levels and activity of the glutamate transporter EAAT2 (GLT-1) in mice. |
プロトコル(参考用のみ)
| 細胞アッセイ | 細胞株 | PC3 cells |
|---|---|---|
| 濃度 | 10-50 μM | |
| 反応時間 | 16 h | |
| 実験の流れ | Cytotoxicity tests are carried out using WST-1, a fluorescent cell proliferation reagent. The assay is based on cleavage of the tetrazolium salt WST-1 by active mitochondria to produce a soluble colored formazan salt. The cells are plated at 1 × l04 in 96-well microtiter plates. Twenty-four hours after plating, at 70% confluence the growth medium is removed and replaced with the test solutions (100 ìl). After 16-hr exposure, the reaction medium (in the presence or absence of 10-50 μM this compound) is removed, the cells are washed twice with culture medium, then 100 μl culture medium and 10 μl WST-1 are added to each well. The cells are incubated for 2 hrs at 37 °C in a humidified atmosphere with 5% CO2, then the microplate is thoroughly shaken for 1 min and the absorbance is measured at 450 nm using a microtiter reader. |
|
| 動物実験 | 動物モデル | Male Swiss albino mice |
| 投薬量 | 1 and 2 mg/animal/day | |
| 投与方法 | oral |
参考
|
Selleckの高級品が、幾つかの出版された研究調査結果(以下を含む)で使われた:
| Gut microbial Nordihydroguaiaretic acid suppresses macrophage pyroptosis to regulate epithelial homeostasis and inflammation [ Gut Microbes, 2025, 17(1):2518338] | PubMed: 40596758 |
長期の保管のために-20°Cの下で製品を保ってください。
人間や獣医の診断であるか治療的な使用のためにでない。
各々の製品のための特定の保管と取扱い情報は、製品データシートの上で示されます。大部分のSelleck製品は、推薦された状況の下で安定です。製品は、推薦された保管温度と異なる温度で、時々出荷されます。長期の保管のために必要とされてそれと異なる温度で、多くの製品は、短期もので安定です。品質を維持するが、夜通しの積荷のために最も経済的な貯蔵状況を用いてあなたの送料を保存する状況の下に、製品が出荷されることを、我々は確実とします。製品の受領と同時に、製品データシートの上で貯蔵推薦に従ってください。
