|
受注:045-509-1970 |
技術サポート:tech@selleck.co.jp 平日9:00〜18:00 1営業日以内にご連絡を差し上げます |
化学情報
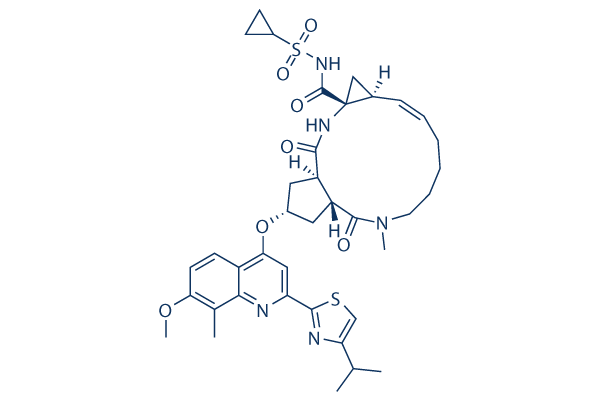
|
Synonyms | TMC435, TMC-435350 | Storage (From the date of receipt) |
3 years -20°C powder 1 years -80°C in solvent |
|||
| 化学式 | C38H47N5O7S2 |
||||||
| 分子量 | 749.94 | CAS No. | 923604-59-5 | ||||
| Solubility (25°C)* | 体外 | DMSO | 100 mg/mL (133.34 mM) | ||||
| Ethanol | 4 mg/mL (5.33 mM) | ||||||
| Water | Insoluble | ||||||
| 体内 (毎回新しく調製した物を用意してください) |
|
||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. |
|||||||
溶剤液(一定の濃度)を調合する
生物活性
| 製品説明 | Simeprevir is a competitive, reversible, macrocyclic, noncovalent hepatitis C virus (HCV) NS3/4A protease inhibitor that acts directly against the hepatitis C virus. It has a medium inhibitory concentration (IC50) <13 nM for all HCV NS3/4A enzymes(genotypes 1a, 1b, 2, 4, 5, and 6), but has an IC50 value of 37 nM for genotype 3. |
|---|---|
| in vitro | Simeprevir exhibits potent inhibition on NS3/4A protease of genotypes 1a, 1b, 2, 4, 5, and 6, with a medium inhibitory concentration (IC50) <13 nM for all HCV NS3/4A enzymes tested. However, the IC50 for genotype 3 is 37 nM. In vitro, this compound is also an inhibitor of bilirubin transporters OATP1B1 and MRP2. It is a more potent inhibitor of OATP1B1 (IC50=720 nM), which is primarily responsible for transporting unconjugated bilirubin, than MRP2 (IC50 around 10,000 nM), primarily a conjugated bilirubin transporter[1]. |
| in vivo | In vivo, simeprevir has a relatively long absorption phase, reaching maximum concentration (Cmax) after 4-6 hours. It is extensively (99.9%) bound to plasma proteins, mainly to albumin. The absolute bioavailability is 44% after a single oral administration. In rats, the liver to blood ratio is 29:1, which means good distribution to the liver. For humans, in preclinical studies, the liver to plasma concentration ratio is really high (ratio of 39). The highest tissue/plasma AUC ratios are observed in the small intestine (ratio of 128). While tissue concentrations of this compound reach peak values within 4 hours postdosing, its concentrations in liver remain above the EC99 for up to 31 hours postdosing, and plasma concentrations are higher than the EC99 at 8 hours and around the EC50 at 24 hours postdosing. The AUC24h of this chemical is increased by 61%-69% when administered with food. It should therefore be taken with food. This compound is also a substrate and inhibitor of P-glycoprotein. It is metabolized by CYP3A4 and eliminated by biliary excretion. It is also an inhibitor of gut cytochrome 3A4 but not hepatic CYP3A4[1]. |
プロトコル(参考用のみ)
参考
|
Selleckの高級品が、幾つかの出版された研究調査結果(以下を含む)で使われた:
| Going Viral: An Investigation into the Chameleonic Behaviour of Antiviral Compounds [ Chemistry, 2022, e202202798.] | PubMed: 36286339 |
| Hepatitis C virus drugs that inhibit SARS-CoV-2 papain-like protease synergize with remdesivir to suppress viral replication in cell culture [ Cell Rep, 2021, 35(7):109133] | PubMed: 33984267 |
| Development of a Cell-Based Luciferase Complementation Assay for Identification of SARS-CoV-2 3CLpro Inhibitors [ Viruses, 2021, 13(2)173] | PubMed: 33498923 |
| Characterization of fluorescent probe substrates to develop an efficient high-throughput assay for neonatal hepatic CYP3A7 inhibition screening [ Sci Rep, 2021, 11(1):19443] | PubMed: 34593846 |
| Fluoxazolevir inhibits hepatitis C virus infection in humanized chimeric mice by blocking viral membrane fusion [ Nat Microbiol, 2020, 10.1038/s41564-020-0781-2] | PubMed: 32868923 |
| Sofosbuvir Activates EGFR-Dependent Pathways in Hepatoma Cells with Implications for Liver-Related Pathological Processes. [ Cells, 2020, 17;9(4) pii: E1003] | PubMed: 32316635 |
| Sofosbuvir Activates EGFR-Dependent Pathways in Hepatoma Cells with Implications for Liver-Related Pathological Processes [ Cells, 2020, 9(4)E1003] | PubMed: 32316635 |
| Hepatitis C Virus Drugs Simeprevir And Grazoprevir Synergize with Remdesivir to Suppress SARS-CoV2 Replication [ BioRxiv, 2020, 10.1101/2020.12.13.422511] | PubMed: none |
長期の保管のために-20°Cの下で製品を保ってください。
人間や獣医の診断であるか治療的な使用のためにでない。
各々の製品のための特定の保管と取扱い情報は、製品データシートの上で示されます。大部分のSelleck製品は、推薦された状況の下で安定です。製品は、推薦された保管温度と異なる温度で、時々出荷されます。長期の保管のために必要とされてそれと異なる温度で、多くの製品は、短期もので安定です。品質を維持するが、夜通しの積荷のために最も経済的な貯蔵状況を用いてあなたの送料を保存する状況の下に、製品が出荷されることを、我々は確実とします。製品の受領と同時に、製品データシートの上で貯蔵推薦に従ってください。
