|
受注:045-509-1970 |
技術サポート:tech@selleck.co.jp 平日9:00〜18:00 1営業日以内にご連絡を差し上げます |
化学情報
|
Synonyms | MK 733 | Storage (From the date of receipt) |
3 years -20°C powder 1 years -80°C in solvent |
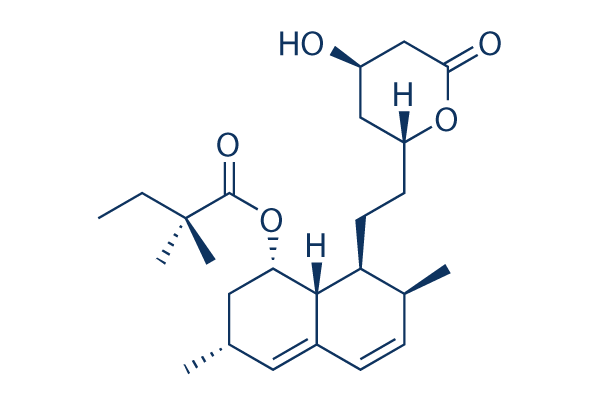
| 化学式 | C25H38O5 |
|||
| 分子量 | 418.57 | CAS No. | 79902-63-9 | |
| Solubility (25°C)* | 体外 | DMSO | 84 mg/mL (200.68 mM) | |
| Ethanol | 84 mg/mL (200.68 mM) | |||
| Water | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. |
||||
溶剤液(一定の濃度)を調合する
生物活性
| 製品説明 | Simvastatin is a competitive inhibitor of HMG-CoA reductase with Ki of 0.1-0.2 nM in cell-free assays. Simvastatin induces ferroptosis, mitophagy, autophagy and apoptosis. |
|---|---|
| in vitro | Prior to use in cell assays, Simvastatin needs to be activated by NaOH in EtOH treatment. This compound inhibits cholesterol synthesis in mouse L-M cell (fibroblast), rat H4II E cell (liver), and human Hep G2 cell (liver) with IC50 of 19.3 nM, 13.3 nM and 15.6 nM, respectively. [1] This compound treatment leads to a dose-dependent increase in serine 473 phosphorylation of Akt within 30 minutes, with maximal phosphorylation occurring at 1.0 µM. It (1.0 μM) enhances phosphorylation of the endogenous Akt substrate endothelial nitric oxide synthase (eNOS), inhibits serum-free media undergo apoptosis and accelerates vascular structure formation. [2] This chemical displays anti-inflammatory effects in vitro. It (10 μM) reduces anti-CD3/anti-CD28 antibody-stimulated proliferation of PB-derived mononuclear cells and synovial fluid cells from rheumatoid arthritis blood, as well as IFN-γ release. This compound (10 μM) suppresses cell-mediated macrophage TNF-γ release induced via cognate interactions by ~30%. [3] |
| in vivo | Simvastatin orally administration inhibits the conversion of radiolabeled acetate to cholesterol with IC50 of 0.2 mg/kg. [1] This compound (4 mg/day) orally administration for 13 weeks to rabbits fed an atherogenci cholesterol-rich diet, returns the cholesterol-induced increases in total cholesterol, LDL-cholesterol and HDL-cholesterol to normal level. [4] This compound (6 mg/kg) produces an increase in LDL receptor-dependent binding and increases the number of hepatic LDL receptors in rabbits fed a diet containing 0.25% cholesterol. [5] This compound influences inflammation independent of its effect on plasma cholesterol level. In cynomolgus monkeys consumed an atherogenic diet, this compound (20 mg/kg/day) induces a 1.3-fold less macrophage content in lesions, and 2-fold less vascular cell adhesion molecule-1, interleukin-1beta, and tissue factor expression, companied by a 2.1-fold increases in lesional smooth muscle cell and collagen content. [6] |
プロトコル(参考用のみ)
| キナーゼアッセイ | HMG-CoA reductase activity assay | |
|---|---|---|
| The total volume of each assay is 95 μL and the reaction mixture contained 10 μL of the inhibiting compound to be tested and 85 μL of the incubating buffer containing 2 mg/mL liver microsomes, 0.1 M KH2PO4, pH 7.2, 5.7 mM dithiothreitol, 10 mM glucose-6-phosphate, 2 U/mL glucose-6-phosphate dehydrogenase, 1 mM NADP, 10 μM miconazole. Control experiments are done without NADPH generating system. All samples are incubated 10 min at 37 ℃ before addition of 5μL of substrate (unlabelled and 14C-HMG-3-hydroxy-3-methyl glutaryl CoA, final concentration 50 μM, 2.5 nCi/nmole). After 30 min at 37 ℃, the reaction is stopped by adding 27 μL 1N HCl and 20 μL of unlabelled mevalonolactone (200 μg/assay). The conversion of mevalonic acid to lactone is performed at room temperature for 60 min. | ||
| 細胞アッセイ | 細胞株 | HUVECs |
| 濃度 | 1 μM | |
| 反応時間 | 30 minutes | |
| 実験の流れ | HUVECs were treated with simvastatin for 30 minutes. |
|
| 動物実験 | 動物モデル | Male Japanese white rabbits |
| 投薬量 | 2.5, 5, & 10 mg/kg | |
| 投与方法 | Oral | |
参考
|
カスタマーフィードバック
-
, , JAMA Oncol, 2015, 1(4):495-504.
-
Data from [Data independently produced by , , Haematologica, 2018, doi: 10.3324/haematol.2018.204701]
-
Data from [Data independently produced by , , Cell Physiol Biochem, 2018, 46(2):618-632]
Selleckの高級品が、幾つかの出版された研究調査結果(以下を含む)で使われた:
| Fructose and glucose from sugary drinks enhance colorectal cancer metastasis via SORD [ Nat Metab, 2025, 7(10):2018-2032] | PubMed: 40973819 |
| Simvastatin overcomes the pPCK1-pLDHA-SPRINGlac axis-mediated ferroptosis and chemo-immunotherapy resistance in AKT-hyperactivated intrahepatic cholangiocarcinoma [ Cancer Commun (Lond), 2025, 10.1002/cac2.70036] | PubMed: 40443016 |
| Coenzyme A protects against ferroptosis via CoAlation of mitochondrial thioredoxin reductase [ J Clin Invest, 2025, e190215] | PubMed: 40694424 |
| Commonly prescribed multi-medication therapies exert sex-specific effects on Alzheimer's disease pathology and metabolomic profiles in AppNL-G-F mice: Implications for personalized therapeutics in aging [ Alzheimers Dement, 2025, 21(3):e70081] | PubMed: 40145346 |
| Macrophages form dendrite-like pseudopods to enhance bacterial ingestion [ EMBO J, 2025, 10.1038/s44318-025-00515-z] | PubMed: 40721684 |
| CRISPR/Cas9-mediated SHP-1-knockout T cells combined with simvastatin enhances anti-tumor activity in humanized-PDX HCC model [ iScience, 2025, 28(4):112266] | PubMed: 40241752 |
| Ferroptosis as a therapeutic vulnerability in MDM2 inhibition in dedifferentiated liposarcoma [ Oncol Lett, 2025, 29(6):269] | PubMed: 40247991 |
| Epstein-Barr Virus-Driven B-Cell Transformation under Germinal Center Hypoxia Requires External Unsaturated Fatty Acids [ Res Sq, 2025, rs.3.rs-6506954] | PubMed: 40313738 |
| The mevalonate pathway couples lipid metabolism to amino acid synthesis via ubiquinone-dependent redox control [ bioRxiv, 2025, 2025.08.10.669191] | PubMed: 40832310 |
| ETS1 Orchestrates a Hybrid EMT Program Driving in vivo Metastasis and Immune Evasion [ bioRxiv, 2025, 2025.07.17.665404] | PubMed: 40777467 |
長期の保管のために-20°Cの下で製品を保ってください。
人間や獣医の診断であるか治療的な使用のためにでない。
各々の製品のための特定の保管と取扱い情報は、製品データシートの上で示されます。大部分のSelleck製品は、推薦された状況の下で安定です。製品は、推薦された保管温度と異なる温度で、時々出荷されます。長期の保管のために必要とされてそれと異なる温度で、多くの製品は、短期もので安定です。品質を維持するが、夜通しの積荷のために最も経済的な貯蔵状況を用いてあなたの送料を保存する状況の下に、製品が出荷されることを、我々は確実とします。製品の受領と同時に、製品データシートの上で貯蔵推薦に従ってください。
