|
受注:045-509-1970 |
技術サポート:[email protected] 平日9:00〜18:00 1営業日以内にご連絡を差し上げます |
化学情報
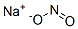
|
Synonyms | N/A | Storage (From the date of receipt) |
3 years -20°C powder 1 years -80°C in solvent |
|||
| 化学式 | HNO2.Na |
||||||
| 分子量 | 69 | CAS No. | 7632-00-0 | ||||
| Solubility (25°C)* | 体外 | DMSO (warmed with 50ºC water bath) | 13 mg/mL (188.4 mM) | ||||
| Water (warmed with 50ºC water bath) | 13 mg/mL (188.4 mM) | ||||||
| Ethanol | Insoluble | ||||||
| 体内 (毎回新しく調製した物を用意してください) |
|
||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. |
|||||||
溶剤液(一定の濃度)を調合する
生物活性
| 製品説明 | Sodium nitrite is a myeloperoxidase inhibitor with IC50 of 1.3 μM.This product is a hazardous chemical (acute toxicity/flammable/skin corrosive). Please use it while wearing a protective face mask, gloves, and clothing. |
|---|---|
| in vitro | Sodium nitrite is well known for its role in inhibiting the growth of Clostridium botulinum by inhibiting iron-sulfur clusters essential to energy metabolism. Sodium nitrite slows chlorination by univalently reducing myeloperoxidase to an inactive form and as a consequence is oxidized to nitrogen dioxide. Myeloperoxidase oxidizes free tyrosine to tyrosyl radicals that exchange with tyrosyl residues in peptides. These peptide radicals then couple with nitrogen dioxide to form 3-nitrotyrosyl residues. With neutrophils, myeloperoxidase-dependent nitration required a high concentration of nitrite (1 mM), is doubled by tyrosine, and increases 4-fold by superoxide dismutase. Superoxide is likely to inhibit nitration by reacting with nitrogen dioxide and/or tyrosyl radicals. [1] Sodium nitrite results in intoxication of white mice with decrease of red cell superoxide dismutase (SOD) and catalase activity. The total activity of glucose-6-phosphate dehydrogenase and dehydrogenase of 6-phosphogluconate as well as the activity of glutathione reductase are higher in the group of mice that receive sodium nitrite in comparison with the control group. [3] |
| in vivo | Peak plasma levels of nitrite are achieved in both sexes approximately 30 min after oral exposure. The model predicts that 10% of the hemoglobin is oxidized to the ferric form after oral doses of 15.9 mg/kg in male rats and 11.0 mg/kg in female rats and after intravenous doses of 8.9 and 7.1 mg/kg in male and female rats, respectively. The t1/2 for recovery from methemoglobinemia is 60 to 120 min depending on dose and route of administration. Replacement of the Vmax of methemoglobin reductase with a value representative of humans predicted a 10% methemoglobinemia following an intravenous dose of 5.8 mg/kg, in close agreement with an observed value of 5.7 mg/kg for humans. [2] |
プロトコル(参考用のみ)
| キナーゼアッセイ | H2O2 uptake assay | |
|---|---|---|
| Reactions are started by adding 10 nM myeloperoxidase to 30 μM H 2 O 2 and varying concentrations of nitrite, in 100 mM phosphate buffer, pH 7.4, containing 20 μM DTPA. Steady state rates of H 2 O 2 loss at 21°C are calculated over the first minute. Data are means and ranges of at least duplicate experiments. | ||
| 細胞アッセイ | 細胞株 | Jurkat cells |
| 濃度 | ~25 μM | |
| 反応時間 | 36 h | |
| 実験の流れ | cell density |
|
| 動物実験 | 動物モデル | Twelve-week old Fischer 344 rats |
| 投薬量 | 20 mg/kg | |
| 投与方法 | tail vein injection | |
参考
長期の保管のために-20°Cの下で製品を保ってください。
人間や獣医の診断であるか治療的な使用のためにでない。
各々の製品のための特定の保管と取扱い情報は、製品データシートの上で示されます。大部分のSelleck製品は、推薦された状況の下で安定です。製品は、推薦された保管温度と異なる温度で、時々出荷されます。長期の保管のために必要とされてそれと異なる温度で、多くの製品は、短期もので安定です。品質を維持するが、夜通しの積荷のために最も経済的な貯蔵状況を用いてあなたの送料を保存する状況の下に、製品が出荷されることを、我々は確実とします。製品の受領と同時に、製品データシートの上で貯蔵推薦に従ってください。
