|
受注:045-509-1970 |
技術サポート:[email protected] 平日9:00〜18:00 1営業日以内にご連絡を差し上げます |
化学情報
|
Synonyms | A 179578 | Storage (From the date of receipt) |
3 years -20°C powder 1 years -80°C in solvent |
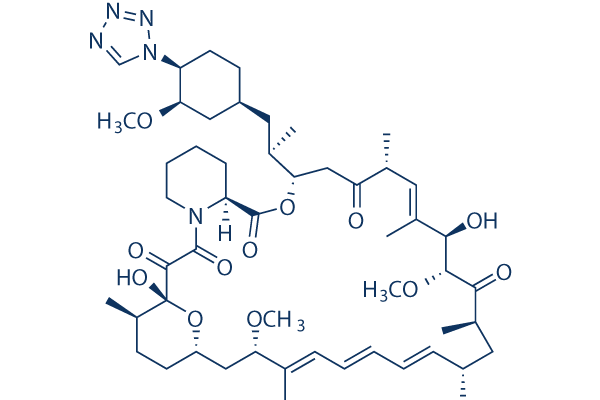
| 化学式 | C52H79N5O12 |
|||
| 分子量 | 966.21 | CAS No. | 221877-54-9 | |
| Solubility (25°C)* | 体外 | DMSO | 100 mg/mL (103.49 mM) | |
| Ethanol | 100 mg/mL (103.49 mM) | |||
| Water | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. |
||||
溶剤液(一定の濃度)を調合する
生物活性
| 製品説明 | Zotarolimus (ABT-578,A 179578) is an analogue of rapamycin, and inhibits FKBP-12 binding with IC50 of 2.8 nM. |
|---|---|
| in vitro | Zotarolimus (ABT-578) is a semi-synthetic analogue of rapamycin, made by substituting a tetrazole ring for the native hydroxyl group at position 42 in rapamycin. Zotarolimus is highly effective in inhibiting both smooth muscle cell and endothelial cell proliferation, with IC50 values of 2.9 nM and 2.6 nM, respectively. [1] Zotarolimus is mechanistically similar to sirolimus in having high-affinity binding to the immunophilin FKBP12 and comparable potency for inhibiting in vitro proliferation of both human and rat T cells. Zotarolimus inhibits Con A-induced human T cells and rat T cells proliferation with IC50 of 7.0 nM and 1337 nM respectively. [2] |
| in vivo | Zotarolimus-eluting stents effectively reduce neointima formation in a 28-day, well-characterized swine model of coronary artery restenosis. Zotarolimus appears effective in preventing neointimal thickening, reducing late loss from 1.03 to 0.62 mm with a 47% reduction in TVF compared with bare metal stents (15.4% with the Driver stent to 8.1% with the Endeavor stent). [1] Zotarolimus is efficacious in suppressing adjuvant DTH, EAE, and cardiac allograft rejection with ED50 values of 1.72, 1.17, and 3.71 mg/kg/day, respectively. [2] |
| 特徴 | Zotarolimus has a shorter in vivo half-life and is also demonstrated in rats to have less potent systemic immunosuppression than rapamycin. |
プロトコル(参考用のみ)
| キナーゼアッセイ | Binding Affinity to FKBP12 | |
|---|---|---|
| 96-well microtiter plates are first coated with FKBP-12 CMP-KDO synthetase fusion protein at 10 μg/mL, 100 μL/well for 2-3 h, followed by addition of 50 μL/well of buffer A (2% BSA and 0.2% Tween-20 in D-PBS) for 30-60 min. Microtiter plates are then washed three times with buffer B (0.2% Tween in D-PBS, pH adjusted to 7.4). Fifty microlitres of buffer A (for maximum), 20 μM FK506 in buffer A (for background), or various concentrations of zotarolimus (10 pM-1 μM) in buffer A are added to each well followed by addition of 50 μL of A-79397 (an FK506 analogue)-alkaline phosphatase conjugate in buffer A. Microtiter plates are incubated at room temperature for 2-2.5 h followed by three washes with buffer B. About 100 μL of pNPP (p-nitrophenyl-phosphate) in 0.1 M aminomethylpropanol are added to each well and plates are incubated at room temperature for 90-120 min. Absorbance at 405 nM is read using an ELISA plate | ||
| 細胞アッセイ | 細胞株 | Human coronary artery cells |
| 濃度 | ~1 μM | |
| 反応時間 | 5 days | |
| 実験の流れ | Cell proliferation is assayed by measuring tritiated thymidine incorporation in vitro. Human coronary artery cells (hCa) are seeded into tissue culture flasks for expansion and applied to 96-well plates at desired density in complete media (5000 hCaSMC; 10 000 hCaEC). After 2 days, complete media is replaced with incomplete media to synchronize cells and induce G0 state. Two days later, incomplete media are removed and replaced with complete media (serum/growth factors) to induce G0 to G1 transition. Complete media also contain drug at desired concentrations to determine its effects on cell proliferation. On day 7, 3H-thymidine is added to cells to monitor DNA synthesis, and cells are harvested after overnight incorporation of radioactivity. After an incubation period of 72 h, 25 μL (1 μCi/well) of 3H-thymidine are added to each well. The cells are incubated at 37°C for 16-18 h to allow for incorporation of 3H-thymidine into newly synthesized DNA and the cells harvested onto 96-well plates containing bonded glass fibre filters . The filter plates are air-dried overnight, MicroScint-20 (25 μL) added to each filter well and counted. Drug activity is determined by the inhibition of 3H-thymidine incorporation into newly synthesized DNA relative to cells grown in complete media. |
|
| 動物実験 | 動物モデル | Male Sprague-Dawley rats |
| 投薬量 | 2.5 mg/kg | |
| 投与方法 | intravenous or oral | |
参考
|
Selleckの高級品が、幾つかの出版された研究調査結果(以下を含む)で使われた:
| Assessment of Lipophilicity Parameters of Antimicrobial and Immunosuppressive Compounds [ Molecules, 2023, 28(6)2820] | PubMed: 36985792 |
| Assessment of Lipophilicity Parameters of Antimicrobial and Immunosuppressive Compounds [ Molecules, 2023, 28(6), 2820] | PubMed: None |
| Rapamycin directly activates lysosomal mucolipin TRP channels independent of mTOR. [ PLoS Biol, 2019, 17(5):e3000252] | PubMed: 31112550 |
| Rapamycin directly activates lysosomal mucolipin TRP channels independent of mTOR. [ PLoS Biol, 2019, 17(5):e3000252] | PubMed: 31112550 |
長期の保管のために-20°Cの下で製品を保ってください。
人間や獣医の診断であるか治療的な使用のためにでない。
各々の製品のための特定の保管と取扱い情報は、製品データシートの上で示されます。大部分のSelleck製品は、推薦された状況の下で安定です。製品は、推薦された保管温度と異なる温度で、時々出荷されます。長期の保管のために必要とされてそれと異なる温度で、多くの製品は、短期もので安定です。品質を維持するが、夜通しの積荷のために最も経済的な貯蔵状況を用いてあなたの送料を保存する状況の下に、製品が出荷されることを、我々は確実とします。製品の受領と同時に、製品データシートの上で貯蔵推薦に従ってください。
