
- 阻害剤
- 研究分野別
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- 化合物ライブラリー
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-II
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at info@selleck.co.jp to customize your library.
You could select:
- 抗体
- 新製品
- お問い合わせ
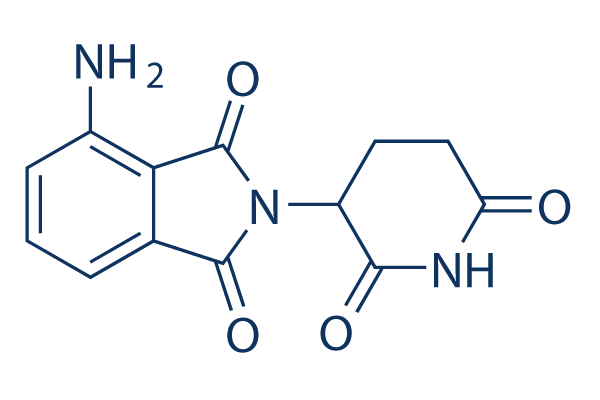
Pomalidomide (CC-4047)
別名:CC-4047
Pomalidomide inhibits LPS-induced TNF-α release with IC50 of 13 nM in PBMCs. Pomalidomide can be utilized in PROTAC as a ligand for targeting E3 ligase and inhibiting the E3 ligase protein cereblon (CRBN). Pomalidomide promotes apoptosis and cell cycle arrest.
CAS No. 19171-19-8
文献中Selleckの製品使用例(122)
製品安全説明書
現在のバッチを見る:
純度:
99.98%
99.98
Pomalidomide (CC-4047)関連製品
| 関連製品 | CC-99282 | もっと見る |
|---|---|---|
| 関連化合物ライブラリー | Kinase Inhibitor Library FDA-approved Drug Library Natural Product Library Bioactive Compound Library-I Highly Selective Inhibitor Library | もっと見る |
E3 ligase Ligand阻害剤の選択性比較
Cell Data
| Cell Lines | Assay Type | Concentration | Incubation Time | 活性情報 | PMID |
|---|---|---|---|---|---|
| SH-SY5Y | Apoptosis Assay | 25 μg/mL | 1 h | causes statistically significant reduction in both CPF- and CPF+CM-induced apoptosis | 24975276 |
| RPMI8226 | Function Assay | 0.1-10 μM | 4 h | increases VEGF mRNA expression | 25053990 |
| OPM2 | Function Assay | 10 μM | 48 h | strengthens cytoplasmic-nuclear shuttling of mTOR and p-mTOR protein | 26097872 |
| RPMI8226 | Function Assay | 10 μM | 48 h | strengthens cytoplasmic-nuclear shuttling of mTOR and p-mTOR protein | 26097872 |
| OPM2 | Growth Inhibition Assay | 0.01-50 μM | 48 h | IC50=10 μM | 26097872 |
| RPMI8226 | Growth Inhibition Assay | 0.01-50 μM | 48 h | IC50=8 μM | 26097872 |
| APK-1 | Growth Inhibition Assay | 39-1250 nM | 5 d | IC50=226 nM, inhibits cell viability dose dependently | 26119939 |
| BCP-1 | Growth Inhibition Assay | 39-1250 nM | 5 d | IC50=396 nM, inhibits cell viability dose dependently | 26119939 |
| BC-1 | Growth Inhibition Assay | 39-1250 nM | 5 d | IC50=744 nM, inhibits cell viability dose dependently | 26119939 |
| UMPEL-3 | Growth Inhibition Assay | 39-1250 nM | 5 d | IC50=111 nM, inhibits cell viability dose dependently | 26119939 |
| UMPEL-1 | Growth Inhibition Assay | 39-1250 nM | 5 d | IC50=32 nM, inhibits cell viability dose dependently | 26119939 |
| VG-1 | Growth Inhibition Assay | 39-1250 nM | 5 d | IC50=101 nM, inhibits cell viability dose dependently | 26119939 |
| JSC-1 | Growth Inhibition Assay | 39-1250 nM | 5 d | IC50=34 nM, inhibits cell viability dose dependently | 26119939 |
| BCBL-1 | Growth Inhibition Assay | 39-1250 nM | 5 d | IC50=74 nM, inhibits cell viability dose dependently | 26119939 |
| BC-3 | Growth Inhibition Assay | 39-1250 nM | 5 d | IC50=107 nM, inhibits cell IC50=107 nM, viability dose dependently | 26119939 |
| R-CD38 | Cytotoxicity Assay | 10 μM | 24 h | potently augments direct and indirect MM cell killing by SAR | 26338273 |
| J-CD38 | Cytotoxicity Assay | 10 μM | 24 h | potently augments direct and indirect MM cell killing by SAR | 26338273 |
| MOLP-8 | Cytotoxicity Assay | 10 μM | 24 h | potently augments direct and indirect MM cell killing by SAR | 26338273 |
| JJN3 | Growth Inhibition Assay | 0.1-100 μM | 72 h | inhibits cell growth slightly | 23178378 |
| XG-1 | Growth Inhibition Assay | 0.1-100 μM | 72 h | inhibits cell growth | 23178378 |
| CD138+ | Growth Inhibition Assay | 0.1-100 μM | 72 h | inhibits cell growth | 23178378 |
| XG-1 | Function Assay | 2/100 μM | 24 h | inhibits CCL3/MIP-1α mRNA expression | 23178378 |
| U266 | Growth Inhibition Assay | 0.01-10 μM | 48 h | inhibits cell growth dose dependently | 22552008 |
| CRBN60 | Growth Inhibition Assay | 0.01-10 μM | 48 h | inhibits cell growth dose dependently | 22552008 |
| CRNB75 | Growth Inhibition Assay | 0.01-10 μM | 48 h | inhibits cell growth dose dependently | 22552008 |
| MM.1S | Growth Inhibition Assay | 0.01-10 μM | 48 h | significantly inhibits proliferation at concentrations as low as 0.01μM | 21389327 |
| OPM2 | Growth Inhibition Assay | 0.01-10 μM | 48 h | significantly inhibits proliferation at concentrations as low as 0.01μM | 21389327 |
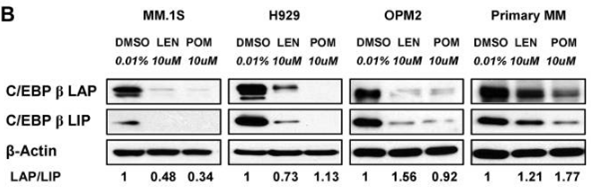
| MM.1S | Function Assay | 10 μM | 72 h | significantly decreases the protein level of C/EBPβ isoforms | 21389327 |
| H929 | Function Assay | 10 μM | 72 h | significantly decreases the protein level of C/EBPβ isoforms | 21389327 |
| OPM2 | Function Assay | 10 μM | 72 h | significantly decreases the protein level of C/EBPβ isoforms | 21389327 |
| CT26 | Function Assay | 1/10 μM | 24 h | reduces the numbers of live colonies | 19638977 |
| DF15 | Function assay | 0.01 to 1 uM | 5 hrs | Induction of cereblon-mediated aiolos degradation in human DF15 cells at 0.01 to 1 uM after 5 hrs by immunoblot analysis | 28425720 |
| OPM2 | Function assay | 0.01 to 1 uM | 5 hrs | Induction of cereblon-mediated ikaros degradation in human OPM2 cells at 0.01 to 1 uM after 5 hrs by immunoblot analysis | 28425720 |
| DF15 | Function assay | 0.01 to 1 uM | 5 hrs | Induction of cereblon-mediated ikaros degradation in human DF15 cells at 0.01 to 1 uM after 5 hrs by immunoblot analysis | 28425720 |
| OPM2 | Function assay | 0.01 to 1 uM | 5 hrs | Induction of cereblon-mediated aiolos degradation in human OPM2 cells at 0.01 to 1 uM after 5 hrs by immunoblot analysis | 28425720 |
| T-cells | Function assay | 2 to 3 days | Inhibition of IL-2 production in human T cells measured after 2 to 3 days by ELISA, EC50 = 0.008 μM. | 23168019 | |
| DF15 | Function assay | 4 hrs | Induction of cereblon-mediated aiolos degradation in human DF15 cells expressing ePL-tagged aiolos after 4 hrs by luminometric analysis, EC50 = 0.022 μM. | 28425720 | |
| DF15 | Function assay | 4 hrs | Induction of cereblon-mediated ikaros degradation in human DF15 cells expressing ePL-tagged ikaros after 4 hrs by luminometric analysis, EC50 = 0.024 μM. | 28425720 | |
| DF15 | Function assay | 4 hrs | Induction of CRL4/CRBN ubiquitin ligase-mediated aiolos degradation in human DF15 cells expressing pLOC-ePL-tagged aiolos after 4 hrs by luminescence based beta-galactosidase enzyme fragmentation complementation assay, EC50 = 0.027 μM. | 28358507 | |
| NAMALWA | Antiproliferative assay | 72 hrs | Antiproliferative activity against human NAMALWA cells assessed as inhibition of [3H]thymidine incorporation after 72 hrs by scintillation counting, IC50 = 0.03 μM. | 23168019 | |
| HeLa | Function assay | Inhibition of IL-1-alpha-induced NF-kappaB activation in HeLa cells assessed as blocking of p50/p65 nuclear translocation, IC50 = 1.27 μM. | 17845850 | ||
| 他の多くの細胞株試験データをご覧になる場合はこちらをクリックして下さい | |||||
生物活性
| 製品説明 | Pomalidomide inhibits LPS-induced TNF-α release with IC50 of 13 nM in PBMCs. Pomalidomide can be utilized in PROTAC as a ligand for targeting E3 ligase and inhibiting the E3 ligase protein cereblon (CRBN). Pomalidomide promotes apoptosis and cell cycle arrest. | ||||
|---|---|---|---|---|---|
| 特性 | A derivative of thalidomide and up to 10,000 times more potent than thalidomide. | ||||
| Targets |
|
| In Vitro | ||||
| In vitro |
Pomalidomide inhibits lipopolysaccharide (LPS) stimulated TNF-alpha release in human PBMC and in human whole blood with IC50 values of 13 nM and 25 nM, respectively. This compound inhibits the growth of T regulatory cells which is stimulated by IL-2 with an IC50 of ~1 μM. Treatment with this chemical (6.4 nM-10 μM) increases the production of IL-2 in human peripheral blood T cells, and is slightly more potent in the CD4+ subset than in the CD8+ subset. It is significantly more potent than CC-5013 at elevating IL-2, IL-5, and IL-10 levels, but only slightly more potent than CC-5013 at elevating IFN-γ levels. This agent enhances SEE and Raji cells induced AP-1 transcriptional activity in Jurkat cells in a dose-dependent manner, with a maximal enhancement of 4-fold at 1 μM. Exposure of Raji cells to various concentrations of this compound (2.5-40 μg/mL) for 48 hours leads to a significant decrease in cell proliferation and DNA synthesis. There is a reduction of ~40% compared to vehicle-treated controls. |
|||
|---|---|---|---|---|
| Kinase Assay | Inhibition of TNF-α synthesis | |||
| TNF-α inhibitory activity is measured in lipopolysacharide (LPS) stimulated PBMC. Pomalidomide is added to human PBMCs 1 hour prior to the addition of LPS (1 μg/mL) and incubation continued for an additional 18-20 hours. Supernatants are then harvested, and the concentration of TNF-α in the supernatants is determined by ELISA. The concentration of this compound that inhibits TNF- production by 50% (IC50) is calculated by nonlinear regression analysis. The human whole blood TNF- inhibition assay is run in a similar fashion to the PBMC assay except that heparinized fresh human whole blood is plated directly into microtiter plates. | ||||
| 細胞実験 | 細胞株 | Raji, SU-DHL-4 and SU-DHL-10 cell lines | ||
| 濃度 | Dissolved in DMSO, final concentrations 2.5-40 μg/mL | |||
| 反応時間 | 24 or 48 hours | |||
| 実験の流れ | For assessment of cell apoptosis, Lymphoma cell lines are exposed to Pomalidomide (5 μg/mL) for 24 hours or 48 hours. The cells are stained with FITC-labeled Annexin V and propidium iodine. Cell apoptosis is analyzed by multicolor flow cytometric analysis using a fluorescence-activated cell sorter/FACStar Plus flow cytometer. Cells are scored as apoptotic if they are Annexin V–positive and propidium iodine–negative/positive (early and late apoptosis, respectively). For determination of cell proliferation, the Lymphoma cell lines are exposed to this compound (2.5, 5, 10, 20, and 40 μg/mL) for 24 hours or 48 hours. 1 μCi per well (96-well plate) of [3H]-thymidine is added and cells are incubated for another 18 hours. Cells are then harvested using the Harvest system into the 96-well glass filters and [3H]-thymidine uptake is measured using an automated scintillation counter. |
|||
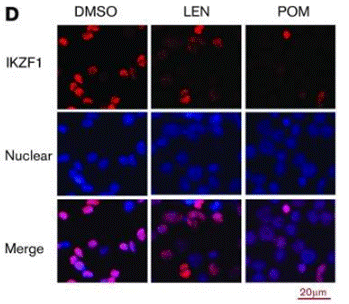
| 実験結果図 | Methods | Biomarkers | 結果図 | PMID |
| Western blot | CEBPβ IKZF1 / IKZF3 / UBE2G1 / CRBN |
|
21389327 | |
| Immunofluorescence | IKZF1 |
|
29496670 | |
| In Vivo | ||
| In Vivo |
Pomalidomide enhances the antitumor effect of rituximab against B-cell lymphomas in severe combined immunodeficient mice. Administration of this compound in combination with rituximab, gives the mice a median survival period of 74 days compared with 58 days of CC5013/rituximab treatment and 45 days of rituximab nonotherapy. The synergistic effect of this compound and rituximab can be completely abrogated by depletion of NK cells, supporting the proposal that NK cell expansion is one mechanism by which this compound may augment rituximab antitumor activity. |
|
|---|---|---|
| 動物実験 | 動物モデル | Disseminated lymphoma-bearing SCID mice |
| 投与量 | 0.5 mg/kg | |
| 投与経路 | Injection i.p. | |
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT04902443 | Recruiting | Viral Associated Malignancies|Kaposi Sarcoma|EBV/KSHV-associated Lymphomas |
National Cancer Institute (NCI)|National Institutes of Health Clinical Center (CC) |
December 10 2021 | Phase 1 |
|
化学情報
| 分子量 | 273.24 | 化学式 | C13H11N3O4 |
| CAS No. | 19171-19-8 | SDF | Download Pomalidomide (CC-4047) SDFをダウンロードする |
| Smiles | C1CC(=O)NC(=O)C1N2C(=O)C3=C(C2=O)C(=CC=C3)N | ||
| 保管 | |||
|
In vitro |
DMSO : 100 mg/mL ( (365.97 mM); 吸湿したDMSOは溶解度を減少させます。新しいDMSOをご使用ください。) Water : Insoluble Ethanol : Insoluble |
モル濃度計算器 |
|
in vivo Add solvents to the product individually and in order. |
投与溶液組成計算機 | |||||
実験計算
投与溶液組成計算機(クリア溶液)
ステップ1:実験データを入力してください。(実験操作によるロスを考慮し、動物数を1匹分多くして計算・調製することを推奨します)
mg/kg
g
μL
匹
ステップ2:投与溶媒の組成を入力してください。(ロット毎に適した溶解組成が異なる場合があります。詳細については弊社までお問い合わせください)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
計算結果:
投与溶媒濃度: mg/ml;
DMSOストック溶液調製方法: mg 試薬を μL DMSOに溶解する(濃度 mg/mL, 注:濃度が当該ロットのDMSO溶解度を超える場合はご連絡ください。 )
投与溶媒調製方法:Take μL DMSOストック溶液に μL PEG300,を加え、完全溶解後μL Tween 80,を加えて完全溶解させた後 μL ddH2O,を加え完全に溶解させます。
投与溶媒調製方法:μL DMSOストック溶液に μL Corn oil,を加え、完全溶解。
注意:1.ストック溶液に沈殿、混濁などがないことをご確認ください;
2.順番通りに溶剤を加えてください。次のステップに進む前に溶液に沈殿、混濁などがないことを確認してから加えてください。ボルテックス、ソニケーション、水浴加熱など物理的な方法で溶解を早めることは可能です。
技術サポート
ストックの作り方、阻害剤の保管方法、細胞実験や動物実験の際に注意すべき点など、製品を取扱う時に問い合わせが多かった質問に対しては取扱説明書でお答えしています。
他に質問がある場合は、お気軽にお問い合わせください。
* 必須
よくある質問(FAQ)
質問1:
Is the formulation S1567 in 1% DMSO+30% polyethylene glycol+1% Tween 80 suitable for its oral administration?
回答
Its suspension in 1% DMSO+30% polyethylene glycol+1% Tween 80 is for oral gavage.
質問2:
I would like to know if it is racemic or optically active?
回答
Our S1567 is racemic.
