- 阻害剤
- 研究分野別
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- 化合物ライブラリー
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-II
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at info@selleck.co.jp to customize your library.
You could select:
- 抗体
- 新製品
- お問い合わせ
Romidepsin (FK228)
別名:FK228, Depsipeptide, FR 901228, NSC 630176,
ロミデプシン (Romidepsin (FK228, Depsipeptide, FR 901228, NSC 630176)) は強力な HDAC1 および HDAC2 阻害剤 (無細胞アッセイで IC50 = 36 nM/47 nM) です。 ロミデプシン (FK228/Depsipeptide) は、神経芽腫腫瘍細胞 (neuroblastoma tumor cells) の増殖を制御し、アポトーシス (apoptosis) を誘導します。
CAS No. 128517-07-7
文献中Selleckの製品使用例(235)
製品安全説明書
現在のバッチを見る:
純度:
99.82%
99.82
Romidepsin (FK228)関連製品
シグナル伝達経路
HDAC阻害剤の選択性比較
Cell Data
| Cell Lines | Assay Type | Concentration | Incubation Time | 活性情報 | PMID |
|---|---|---|---|---|---|
| A549 | Growth Inhibition Assay | 5 nM | 24/48/72 h | inhibits cell growth time-dependently | 22106282 |
| LNCaP | Growth Inhibition Assay | 5 nM | 24/48/72 h | inhibits cell growth time-dependently | 22106282 |
| HFS | Growth Inhibition Assay | 5 nM | 24/48/72 h | inhibits cell growth time-dependently | 22106282 |
| CO115 | Growth Inhibition Assay | 5 nM-50 μM | 24 h | inhibits cell growth in a concentration-dependent manner | 22924958 |
| RKO | Growth Inhibition Assay | 5 nM-50 μM | 24 h | inhibits cell growth in a concentration-dependent manner | 22924958 |
| HCT116 | Growth Inhibition Assay | 5 nM-50 μM | 24 h | inhibits cell growth in a concentration-dependent manner | 22924958 |
| NCI/ADR-RES | Growth Inhibition Assay | 1–20nM | 72 h | reduces cell viability alone and combined with cisplatin | 23010348 |
| OVCAR-8 | Growth Inhibition Assay | 1–20nM | 72 h | reduces cell viability alone and combined with cisplatin | 23010348 |
| Brca1 Null | Growth Inhibition Assay | 1–20nM | 72 h | reduces cell viability alone and combined with cisplatin | 23010348 |
| Brca1 WT | Growth Inhibition Assay | 1–20nM | 72 h | reduces cell viability alone and combined with cisplatin | 23010348 |
| SKOV-3 | Growth Inhibition Assay | 1–20nM | 72 h | reduces cell viability alone and combined with cisplatin | 23010348 |
| DpP75 | Apoptosis Assay | 1/10/100 nM | 48 h | induces blunt apoptosis | 23532732 |
| DpVp50 | Apoptosis Assay | 1/10/100 nM | 48 h | induces blunt apoptosis | 23532732 |
| DpVp35 | Apoptosis Assay | 1/10/100 nM | 48 h | induces blunt apoptosis | 23532732 |
| HuT78 | Apoptosis Assay | 1/10/100 nM | 48 h | induces apoptosis at 1 nM | 23532732 |
| A549 | Growth Inhibition Assay | 10–100 nM | 24/36/48 h | inhibits cell growth in both concentration- and time-dependent manner | 24485799 |
| MS-275 | Growth Inhibition Assay | 0.625-10nM | 48 h | induces a significantly stronger inhibition of cell proliferation co-treated with bortezomib | 24771510 |
| HA | Growth Inhibition Assay | 0.625-10nM | 48 h | induces a significantly stronger inhibition of cell proliferation co-treated with bortezomib | 24771510 |
| ACH-2 | Function Assay | 1-9 nM | 24 h | induces HIV-1 Env expression | 25149467 |
| HCT116 | Growth Inhibition Assay | 5-5000 nM | 24 h | induces cell death in a concentration-dependent manner | 25492515 |
| U2932/EBV | Cell Viability Assay | 2.5-15 nM | 72 h | induces cytotoxicity in a concentration-dependent manner | 25790907 |
| LY7/EBV | Cell Viability Assay | 2.5-15 nM | 72 h | induces cytotoxicity in a concentration-dependent manner | 25790907 |
| Farage | Cell Viability Assay | 2.5-15 nM | 72 h | induces cytotoxicity in a concentration-dependent manner | 25790907 |
| OCI-LY7 | Cell Viability Assay | 2.5-15 nM | 72 h | induces cytotoxicity in a concentration-dependent manner | 25790907 |
| U2932 | Cell Viability Assay | 2.5-15 nM | 72 h | induces cytotoxicity in a concentration-dependent manner | 25790907 |
| SU-DHL4 | Cell Viability Assay | 2.5-15 nM | 72 h | induces cytotoxicity in a concentration-dependent manner | 25790907 |
| THJ-16T | Growth Inhibition Assay | 1 nM | 24 h | inhibits cell growth | 20810568 |
| HCT116 | Function Assay | 20 nM | 8 h | modulates transcript levels for hundreds of genes in either direction | 20739454 |
| B104 | Function Assay | 2 nM | 24/48/72 h | increases the surface expression of CD20 | 20686505 |
| HL-60 | Cytotoxicity Assay | 1-500 nM | 24 h | induces cytotoxicity in a concentration-dependent manner | 20624163 |
| HP100 | Cytotoxicity Assay | 1-500 nM | 24 h | induces cytotoxicity in a concentration-dependent manner | 20624163 |
| HL-60 | Function Assay | 10 nM | 4/6/16 h | induces the generation of hydrogen peroxide from 4h | 20624163 |
| HP100 | Function Assay | 10 nM | 4/6/16 h | induces the generation of hydrogen peroxide from 4h | 20624163 |
| HL-60 | Function Assay | 10-500 nM | 4 h | decreases the histone deacetylase (HDAC) activity | 20624163 |
| HP100 | Function Assay | 10-500 nM | 4 h | decreases the histone deacetylase (HDAC) activity | 20624163 |
| SKOV-3 | Growth Inhibition Assay | 4/8/16 nM | 48 h | inhibits cell growth in a concentration-dependent manner | 20404564 |
| OVCAR-3 | Growth Inhibition Assay | 4/8/16 nM | 48 h | inhibits cell growth in a concentration-dependent manner | 20404564 |
| HBL-2 | Growth Inhibition Assay | 2-10 nM | 24 h | IC50=4.3 nM | 20068080 |
| Jeko-1 | Growth Inhibition Assay | 2-50 nM | 24 h | IC50=11 nM | 20068080 |
| Granta-519 | Growth Inhibition Assay | 5-40 nM | 24 h | IC50=58.5 nM | 20068080 |
| L1236 | Cytotoxicity Assay | 1 nM-100 μM | 48 h | EC50=0.07 μM | 19233470 |
| L428 | Cytotoxicity Assay | 1 nM-100 μM | 48 h | EC50=0.43 μM | 19233470 |
| KM-H2 | Cytotoxicity Assay | 1 nM-100 μM | 48 h | EC50=0.58 μM | 19233470 |
| L540Cy | Cytotoxicity Assay | 1 nM-100 μM | 48 h | EC50=0.16 μM | 19233470 |
| G401 | Function Assay | 10 nM | 24/48/72 h | increases CDKN1C expression | 19221586 |
| STM91-01 | Function Assay | 10 nM | 24/48/72 h | increases CDKN1C expression | 19221586 |
| SJSC | Function Assay | 10 nM | 24/48/72 h | increases CDKN1C expression | 19221586 |
| BT16 | Function Assay | 10 nM | 24/48/72 h | increases CDKN1C expression | 19221586 |
| NCI-H23 | Antitumor assay | 1.6 to 2.4 mg/kg | up to 5 days | Antitumor activity against human NCI-H23 cells implanted intraperitoneally in mouse assessed as inhibition of tumor cell growth at 1.6 to 2.4 mg/kg, ip administered on day 1 to 4 measured on day 5 by hollow fiber assay | 21967146 |
| NCI-H522 | Antitumor assay | 1.6 to 2.4 mg/kg | up to 5 days | Antitumor activity against human NCI-H522 cells implanted intraperitoneally in mouse assessed as inhibition of tumor cell growth at 1.6 to 2.4 mg/kg, ip administered on day 1 to 4 measured on day 5 by hollow fiber assay | 21967146 |
| OVCAR5 | Antitumor assay | 1.6 to 2.4 mg/kg | up to 5 days | Antitumor activity against human OVCAR5 cells implanted intraperitoneally in mouse assessed as inhibition of tumor cell growth at 1.6 to 2.4 mg/kg, ip administered on day 1 to 4 measured on day 5 by hollow fiber assay | 21967146 |
| SW620 | Antitumor assay | 1.6 to 2.4 mg/kg | up to 5 days | Antitumor activity against human SW620 cells implanted intraperitoneally in mouse assessed as inhibition of tumor cell growth at 1.6 to 2.4 mg/kg, ip administered on day 1 to 4 measured on day 5 by hollow fiber assay | 21967146 |
| MDA-MB-435 | Antitumor assay | 1.6 to 2.4 mg/kg | up to 5 days | Antitumor activity against human MDA-MB-435 cells implanted intraperitoneally in mouse assessed as inhibition of tumor cell growth at 1.6 to 2.4 mg/kg, ip administered on day 1 to 4 measured on day 5 by hollow fiber assay | 21967146 |
| COLO205 | Antitumor assay | 1.6 to 2.4 mg/kg | up to 5 days | Antitumor activity against human COLO205 cells implanted intraperitoneally in mouse assessed as inhibition of tumor cell growth at 1.6 to 2.4 mg/kg, ip administered on day 1 to 4 measured on day 5 by hollow fiber assay | 21967146 |
| LOXIMVI | Antitumor assay | 1.6 to 2.4 mg/kg | up to 5 days | Antitumor activity against human LOXIMVI cells implanted intraperitoneally in mouse assessed as inhibition of tumor cell growth at 1.6 to 2.4 mg/kg, ip administered on day 1 to 4 measured on day 5 by hollow fiber assay | 21967146 |
| UACC62 | Antitumor assay | 1.6 to 2.4 mg/kg | up to 5 days | Antitumor activity against human UACC62 cells implanted intraperitoneally in mouse assessed as inhibition of tumor cell growth at 1.6 to 2.4 mg/kg, ip administered on day 1 to 4 measured on day 5 by hollow fiber assay | 21967146 |
| MDA-MB-231 | Antitumor assay | 1.6 to 2.4 mg/kg | up to 5 days | Antitumor activity against human MDA-MB-231 cells implanted intraperitoneally in mouse assessed as inhibition of tumor cell growth at 1.6 to 2.4 mg/kg, ip administered on day 1 to 4 measured on day 5 by hollow fiber assay | 21967146 |
| SF295 | Antitumor assay | 1.6 to 2.4 mg/kg | up to 5 days | Antitumor activity against human SF295 cells implanted intraperitoneally in mouse assessed as inhibition of tumor cell growth at 1.6 to 2.4 mg/kg, ip administered on day 1 to 4 measured on day 5 by hollow fiber assay | 21967146 |
| U251 | Antitumor assay | 1.6 to 2.4 mg/kg | up to 5 days | Antitumor activity against human U251 cells implanted intraperitoneally in mouse assessed as inhibition of tumor cell growth at 1.6 to 2.4 mg/kg, ip administered on day 1 to 4 measured on day 5 by hollow fiber assay | 21967146 |
| OVRAC3 | Antitumor assay | 1.6 to 2.4 mg/kg | up to 5 days | Antitumor activity against human OVRAC3 cells implanted intraperitoneally in mouse assessed as inhibition of tumor cell growth at 1.6 to 2.4 mg/kg, ip administered on day 1 to 4 measured on day 5 by hollow fiber assay | 21967146 |
| 11z | Kinase Assay | 3-100 nM | reduces HDAC enzymatic activity (IC50 = 6.5 ± 0.6 nmol/L) | 20605144 | |
| ST486 | Apoptosis Assay | 6 h | induces extensive apoptosis | 23966164 | |
| Ramos | Apoptosis Assay | 6 h | induces extensive apoptosis | 23966164 | |
| DG75 | Apoptosis Assay | 6 h | induces no apoptosis | 23966164 | |
| CA46 | Apoptosis Assay | 6 h | induces blunt apoptosis | 23966164 | |
| U266 | Growth Inhibition Assay | 24/48 h | EC50s=10 nM; 48 h | 24030150 | |
| RPMI-8226 | Growth Inhibition Assay | 24/48 h | EC50s=1.8 nM; 48 h | 24030150 | |
| OPM-2 | Growth Inhibition Assay | 24/48 h | EC50s=1 nM; 48 h | 24030150 | |
| JJN3 | Growth Inhibition Assay | 24/48 h | EC50<1 nM; 48 h | 24030150 | |
| CD4 T | Growth Inhibition Assay | 48 h | CC50=107±126 nM | 24722454 | |
| CD4 T | Growth Inhibition Assay | 48 h | EC50=4.5±1.0 nM | 24722454 | |
| A498 | Cytotoxicity assay | 6 days | Cytotoxicity against human A498 cells after 6 days by MTT assay, TGI = 0.028 μM. | 21967146 | |
| COLO205 | Cytotoxicity assay | 6 days | Cytotoxicity against human COLO205 cells after 6 days by MTT assay, TGI = 0.031 μM. | 21967146 | |
| U251 | Cytotoxicity assay | 6 days | Cytotoxicity against human U251 cells after 6 days by MTT assay, TGI = 0.031 μM. | 21967146 | |
| RXF393 | Cytotoxicity assay | 6 days | Cytotoxicity against human RXF393 cells after 6 days by MTT assay, TGI = 0.062 μM. | 21967146 | |
| MDA-MB-468 | Cytotoxicity assay | 6 days | Cytotoxicity against human MDA-MB-468 cells after 6 days by MTT assay, TGI = 0.09 μM. | 21967146 | |
| COLO205 | Cytotoxicity assay | 6 days | Cytotoxicity against human COLO205 cells after 6 days by MTT assay, LC50 = 0.092 μM. | 21967146 | |
| UACC257 | Cytotoxicity assay | 6 days | Cytotoxicity against human UACC257 cells after 6 days by MTT assay, TGI = 0.095 μM. | 21967146 | |
| U251 | Cytotoxicity assay | 6 days | Cytotoxicity against human U251 cells after 6 days by MTT assay, LC50 = 0.13 μM. | 21967146 | |
| A498 | Cytotoxicity assay | 6 days | Cytotoxicity against human A498 cells after 6 days by MTT assay, LC50 = 0.2 μM. | 21967146 | |
| NCI-H322M | Cytotoxicity assay | 6 days | Cytotoxicity against human NCI-H322M cells after 6 days by MTT assay, TGI = 0.23 μM. | 21967146 | |
| SF295 | Cytotoxicity assay | 6 days | Cytotoxicity against human SF295 cells after 6 days by MTT assay, TGI = 0.25 μM. | 21967146 | |
| RXF393 | Cytotoxicity assay | 6 days | Cytotoxicity against human RXF393 cells after 6 days by MTT assay, LC50 = 0.3 μM. | 21967146 | |
| NCI60 | Cytotoxicity assay | 6 days | Cytotoxicity against human NCI60 cells after 6 days by MTT assay, TGI = 0.33 μM. | 21967146 | |
| SN12C | Cytotoxicity assay | 6 days | Cytotoxicity against human SN12C cells after 6 days by MTT assay, TGI = 0.34 μM. | 21967146 | |
| MDA-MB-231 | Cytotoxicity assay | 6 days | Cytotoxicity against human MDA-MB-231 cells after 6 days by MTT assay, TGI = 0.35 μM. | 21967146 | |
| ACHN | Cytotoxicity assay | 6 days | Cytotoxicity against human ACHN cells after 6 days by MTT assay, TGI = 0.73 μM. | 21967146 | |
| SN12C | Cytotoxicity assay | 6 days | Cytotoxicity against human SN12C cells after 6 days by MTT assay, LC50 = 1.5 μM. | 21967146 | |
| UO31 | Cytotoxicity assay | 6 days | Cytotoxicity against human UO31 cells after 6 days by MTT assay, TGI = 1.55 μM. | 21967146 | |
| Caki1 | Cytotoxicity assay | 6 days | Cytotoxicity against human Caki1 cells after 6 days by MTT assay, TGI = 1.89 μM. | 21967146 | |
| NCI60 | Cytotoxicity assay | 6 days | Cytotoxicity against human NCI60 cells after 6 days by MTT assay, LC50 = 3.5 μM. | 21967146 | |
| MDA-MB-231 | Cytotoxicity assay | 6 days | Cytotoxicity against human MDA-MB-231 cells after 6 days by MTT assay, LC50 = 4.08 μM. | 21967146 | |
| MDA-MB-468 | Cytotoxicity assay | 6 days | Cytotoxicity against human MDA-MB-468 cells after 6 days by MTT assay, LC50 = 4.14 μM. | 21967146 | |
| ACHN | Cytotoxicity assay | 6 days | Cytotoxicity against human ACHN cells after 6 days by MTT assay, LC50 = 5.37 μM. | 21967146 | |
| UACC257 | Cytotoxicity assay | 6 days | Cytotoxicity against human UACC257 cells after 6 days by MTT assay, LC50 = 5.46 μM. | 21967146 | |
| NCI-H322M | Cytotoxicity assay | 6 days | Cytotoxicity against human NCI-H322M cells after 6 days by MTT assay, LC50 = 9.02 μM. | 21967146 | |
| SF295 | Cytotoxicity assay | 6 days | Cytotoxicity against human SF295 cells after 6 days by MTT assay, LC50 = 10.2 μM. | 21967146 | |
| Caki1 | Cytotoxicity assay | 6 days | Cytotoxicity against human Caki1 cells after 6 days by MTT assay, LC50 = 20.9 μM. | 21967146 | |
| CD4+ T | Function assay | 7 days | Reactivation of latent HIV1 NL4-3-Luc expression in human naive CD4+ T cells treated 7 days post-infection measured after 48 hrs by luminescence plate reader analysis, EC50 = 0.003 μM. | 24495105 | |
| 293T | Function assay | 30 mins | Inhibition of human HDAC1 expressed in human 293T cells using Ac-KGLGK(Ac)-MCA as substrate after 30 mins by fluorescence assay, IC50 = 0.0036 μM. | 24589486 | |
| 293T | Function assay | 30 mins | Inhibition of mouse HDAC6 expressed in human 293T cells using Ac-KGLGK(Ac)-MCA as substrate after 30 mins by fluorescence assay, IC50 = 0.39 μM. | 24589486 | |
| mink Mv1Lu | Function assay | 24 hrs | Increase in human wild type p21 protein expression in mink Mv1Lu cells after 24 hrs by p21 promoter assay in presence of 0.1 mM dithiothreitol, EC1000 = 0.0157 μM. | 24997578 | |
| HCT116 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HCT116 cells after 48 hrs in presence of DTT by MTT assay, IC50 = 0.003 μM. | 25147612 | |
| MOLT4 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MOLT4 cells after 72 hrs by CCK8 assay, GI50 = 0.00206 μM. | 26331334 | |
| DU145 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human DU145 cells after 72 hrs by CCK8 assay, GI50 = 0.00255 μM. | 26331334 | |
| U937 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human U937 cells after 72 hrs by CCK8 assay, GI50 = 0.00341 μM. | 26331334 | |
| HLF | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HLF cells after 72 hrs by CCK8 assay, GI50 = 0.00489 μM. | 26331334 | |
| WI38 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human WI38 cells after 72 hrs by CCK8 assay, GI50 = 0.00539 μM. | 26331334 | |
| U937 | Function assay | 24 hrs | Inhibition of HDAC6 in human U937 cells assessed as reduction of alpha-tubilin acetylation at 5 nM to 5 uM after 24 hrs by Western blot analysis | 26331334 | |
| HCT116 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT116 cells assessed as cell viability incubated for 72 hrs by coulter counter method, IC50 = 0.028 μM. | 26481659 | |
| IGROV1/Pt1 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human IGROV1/Pt1 cells assessed as cell viability incubated for 72 hrs by coulter counter method, IC50 = 0.08 μM. | 26481659 | |
| IGROV1 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human IGROV1 cells assessed as cell viability incubated for 72 hrs by coulter counter method, IC50 = 0.22 μM. | 26481659 | |
| CD4+ T | Growth Inhibition Assay | EC50=3 nM | 24495105 | ||
| HuT-78 | Growth Inhibition Assay | IC50=1.73±0.44 nM | 24954856 | ||
| H526 | Growth Inhibition Assay | IC50=0.15±0.03 nM | 24954856 | ||
| H146 | Growth Inhibition Assay | IC50=0.22±0.07 nM | 24954856 | ||
| EKVX | Growth Inhibition Assay | IC50=1.33±0.34 nM | 24954856 | ||
| H460 | Growth Inhibition Assay | IC50=2.58±0.80 nM | 24954856 | ||
| A549 | Growth Inhibition Assay | IC50=1.26±0.24 nM | 24954856 | ||
| SF-295 | Growth Inhibition Assay | IC50=0.88±0.15 nM | 24954856 | ||
| MDA-MB-435 | Growth Inhibition Assay | IC50=0.90±0.06 nM | 24954856 | ||
| UACC-62 | Growth Inhibition Assay | IC50=0.56±0.16 nM | 24954856 | ||
| LOX-IMVI | Growth Inhibition Assay | IC50=0.87±0.03 nM | 24954856 | ||
| SW620 | Growth Inhibition Assay | IC50=0.93±0.29 nM | 24954856 | ||
| S1 | Growth Inhibition Assay | IC50=7.67±0.29 nM | 24954856 | ||
| HCT116-p21-/- | Growth Inhibition Assay | IC50=1.26±0.37 nM | 24954856 | ||
| HCT116 | Growth Inhibition Assay | IC50=1.00±0.00 nM | 24954856 | ||
| PC3 | Growth Inhibition Assay | IC50=1.65±0.35 nM | 24954856 | ||
| MDA-MB-231 | Growth Inhibition Assay | IC50=0.68±0.14 nM | 24954856 | ||
| SK-BR-3 | Growth Inhibition Assay | IC50=1.00±0.35 nM | 24954856 | ||
| MCF-7 | Growth Inhibition Assay | IC50=1.10±0.20 nM | 24954856 | ||
| MCF-10A | Growth Inhibition Assay | IC50=0.17±0.01 nM | 24954856 | ||
| 697 | Growth Inhibition Assay | IC50 = 2.5 nM | 21538216 | ||
| 697-R | Growth Inhibition Assay | IC50 = 8.6 nM | 21538216 | ||
| HUT78 | Growth Inhibition Assay | IC50=1 nM | 21198545 | ||
| NCI-H1299 | Growth Inhibition Assay | IC50=4.6±0.2 ng/ml | 19179890 | ||
| NCI-2882 | Growth Inhibition Assay | IC50=1.6±0.04 ng/ml | 19179890 | ||
| HCC95 | Growth Inhibition Assay | IC50=2.5±0.05 ng/ml | 19179890 | ||
| NCI-H23 | Growth Inhibition Assay | IC50=2.9±0.2 ng/ml | 19179890 | ||
| NCI-H157 | Growth Inhibition Assay | IC50=1.6±0.02 ng/ml | 19179890 | ||
| NCI-H460 | Growth Inhibition Assay | IC50=2.1±0.07 ng/ml | 19179890 | ||
| NCI-H1975 | Growth Inhibition Assay | IC50=1.3±0.04 ng/ml | 19179890 | ||
| NCI-H820 | Growth Inhibition Assay | IC50=2.4±0.1 ng/ml | 19179890 | ||
| NCI-H1650 | Growth Inhibition Assay | IC50=4.9±0.3 ng/ml | 19179890 | ||
| DTC1 | Growth Inhibition Assay | IC50=0.51 nM | 18566246 | ||
| KAO | Growth Inhibition Assay | IC50=0.91 nM | 18566246 | ||
| SU-CCS-1 | Growth Inhibition Assay | IC50=0.89 nM | 18566246 | ||
| SYO-1 | Growth Inhibition Assay | IC50=0.67 nM | 18566246 | ||
| FUJI | Growth Inhibition Assay | IC50=1.31 nM | 18566246 | ||
| SKNMC | Growth Inhibition Assay | IC50=1.17 nM | 18566246 | ||
| 402-91 | Growth Inhibition Assay | IC50=1.26 nM | 18566246 | ||
| 1765-92 | Growth Inhibition Assay | IC50=1.77 nM | 18566246 | ||
| JN-DSRCT-1 | Growth Inhibition Assay | IC50=1.25 nM | 18566246 | ||
| NMS-2PC | Growth Inhibition Assay | IC50=0.81 nM | 18566246 | ||
| HL60 | Growth Inhibition Assay | IC50=1.86 nM | 18566246 | ||
| A549 | Growth Inhibition Assay | IC50=3.24 nM | 18566246 | ||
| SW480 | Growth Inhibition Assay | IC50=2.69 nM | 18566246 | ||
| MCF7 | Growth Inhibition Assay | IC50=3.55 nM | 18566246 | ||
| PC-3 | Growth Inhibition Assay | IC50=2.51 nM | 18566246 | ||
| MMRU | Growth Inhibition Assay | IC50=2.57 nM | 18566246 | ||
| Hs68 | Growth Inhibition Assay | IC50=>10 nM | 18566246 | ||
| hMSC-001F | Growth Inhibition Assay | IC50=>10 nM | 18566246 | ||
| mammalian cells | Function assay | Inhibition of Histone deacetylase 4 in mammalian cells, IC50 = 0.51 μM. | 14584932 | ||
| mammalian cells | Function assay | Inhibition of Histone deacetylase 1 induced acetylated histone in mammalian cells., EC50 = 36 μM. | 14584932 | ||
| MCF7 | Growth inhibition assay | Growth inhibition of MCF7 cells, IC50 = 0.00075 μM. | 17958342 | ||
| LLC | Function assay | Inhibition of HDAC-mediated HIF-1alpha activity in mouse LLC cells, IC50 = 2 μM. | 22305612 | ||
| NCI-H522 | Growth inhibition assay | Growth inhibition of human NCI-H522 cells by sulforhodamine B colorimetric method, GI50 = 0.0018 μM. | 23313638 | ||
| LOXIMVI | Growth inhibition assay | Growth inhibition of human LOXIMVI cells by sulforhodamine B colorimetric method, GI50 = 0.0025 μM. | 23313638 | ||
| A549 | Growth inhibition assay | Growth inhibition of human A549 cells by sulforhodamine B colorimetric method, GI50 = 0.0026 μM. | 23313638 | ||
| OVCAR5 | Growth inhibition assay | Growth inhibition of human OVCAR5 cells by sulforhodamine B colorimetric method, GI50 = 0.0028 μM. | 23313638 | ||
| MKN74 | Growth inhibition assay | Growth inhibition of human MKN74 cells by sulforhodamine B colorimetric method, GI50 = 0.003 μM. | 23313638 | ||
| NCI-H460 | Growth inhibition assay | Growth inhibition of human NCI-H460 cells by sulforhodamine B colorimetric method, GI50 = 0.003 μM. | 23313638 | ||
| HCT116 | Growth inhibition assay | Growth inhibition of human HCT116 cells by sulforhodamine B colorimetric method, GI50 = 0.0031 μM. | 23313638 | ||
| HCC2998 | Growth inhibition assay | Growth inhibition of human HCC2998 cells by sulforhodamine B colorimetric method, GI50 = 0.0031 μM. | 23313638 | ||
| MKN1 | Growth inhibition assay | Growth inhibition of human MKN1 cells by sulforhodamine B colorimetric method, GI50 = 0.0032 μM. | 23313638 | ||
| SKOV3 | Growth inhibition assay | Growth inhibition of human SKOV3 cells by sulforhodamine B colorimetric method, GI50 = 0.0033 μM. | 23313638 | ||
| HT-29 | Growth inhibition assay | Growth inhibition of human HT-29 cells by sulforhodamine B colorimetric method, GI50 = 0.0033 μM. | 23313638 | ||
| KM12 | Growth inhibition assay | Growth inhibition of human KM12 cells by sulforhodamine B colorimetric method, GI50 = 0.0034 μM. | 23313638 | ||
| DMS114 | Growth inhibition assay | Growth inhibition of human DMS114 cells by sulforhodamine B colorimetric method, GI50 = 0.0036 μM. | 23313638 | ||
| SF539 | Growth inhibition assay | Growth inhibition of human SF539 cells by sulforhodamine B colorimetric method, GI50 = 0.0036 μM. | 23313638 | ||
| U251 | Growth inhibition assay | Growth inhibition of human U251 cells by sulforhodamine B colorimetric method, GI50 = 0.0039 μM. | 23313638 | ||
| SF295 | Growth inhibition assay | Growth inhibition of human SF295 cells by sulforhodamine B colorimetric method, GI50 = 0.004 μM. | 23313638 | ||
| MCF7 | Growth inhibition assay | Growth inhibition of human MCF7 cells by sulforhodamine B colorimetric method, GI50 = 0.0042 μM. | 23313638 | ||
| NCI-H23 | Growth inhibition assay | Growth inhibition of human NCI-H23 cells by sulforhodamine B colorimetric method, GI50 = 0.0046 μM. | 23313638 | ||
| OVCAR3 | Growth inhibition assay | Growth inhibition of human OVCAR3 cells by sulforhodamine B colorimetric method, GI50 = 0.0046 μM. | 23313638 | ||
| MKN7 | Growth inhibition assay | Growth inhibition of human MKN7 cells by sulforhodamine B colorimetric method, GI50 = 0.0049 μM. | 23313638 | ||
| SF268 | Growth inhibition assay | Growth inhibition of human SF268 cells by sulforhodamine B colorimetric method, GI50 = 0.0049 μM. | 23313638 | ||
| MDA-MB-231 | Growth inhibition assay | Growth inhibition of human MDA-MB-231 cells by sulforhodamine B colorimetric method, GI50 = 0.0055 μM. | 23313638 | ||
| OVCAR8 | Growth inhibition assay | Growth inhibition of human OVCAR8 cells by sulforhodamine B colorimetric method, GI50 = 0.0055 μM. | 23313638 | ||
| DMS273 | Growth inhibition assay | Growth inhibition of human DMS273 cells by sulforhodamine B colorimetric method, GI50 = 0.0058 μM. | 23313638 | ||
| DU145 | Growth inhibition assay | Growth inhibition of human DU145 cells by sulforhodamine B colorimetric method, GI50 = 0.006 μM. | 23313638 | ||
| RXF631L | Growth inhibition assay | Growth inhibition of human RXF631L cells by sulforhodamine B colorimetric method, GI50 = 0.0066 μM. | 23313638 | ||
| HBC4 | Growth inhibition assay | Growth inhibition of human HBC4 cells by sulforhodamine B colorimetric method, GI50 = 0.0069 μM. | 23313638 | ||
| SNB75 | Growth inhibition assay | Growth inhibition of human SNB75 cells by sulforhodamine B colorimetric method, GI50 = 0.0072 μM. | 23313638 | ||
| BSY1 | Growth inhibition assay | Growth inhibition of human BSY1 cells by sulforhodamine B colorimetric method, GI50 = 0.0085 μM. | 23313638 | ||
| NCI-H226 | Growth inhibition assay | Growth inhibition of human NCI-H226 cells by sulforhodamine B colorimetric method, GI50 = 0.0089 μM. | 23313638 | ||
| SNB78 | Growth inhibition assay | Growth inhibition of human SNB78 cells by sulforhodamine B colorimetric method, GI50 = 0.0096 μM. | 23313638 | ||
| HBC5 | Growth inhibition assay | Growth inhibition of human HBC5 cells by sulforhodamine B colorimetric method, GI50 = 0.013 μM. | 23313638 | ||
| MKN45 | Growth inhibition assay | Growth inhibition of human MKN45 cells by sulforhodamine B colorimetric method, GI50 = 0.014 μM. | 23313638 | ||
| MKN28 | Growth inhibition assay | Growth inhibition of human MKN28 cells by sulforhodamine B colorimetric method, GI50 = 0.017 μM. | 23313638 | ||
| PC3 | Growth inhibition assay | Growth inhibition of human PC3 cells by sulforhodamine B colorimetric method, GI50 = 0.018 μM. | 23313638 | ||
| ACHN | Growth inhibition assay | Growth inhibition of human ACHN cells by sulforhodamine B colorimetric method, GI50 = 0.02 μM. | 23313638 | ||
| OVCAR4 | Growth inhibition assay | Growth inhibition of human OVCAR4 cells by sulforhodamine B colorimetric method, GI50 = 0.02 μM. | 23313638 | ||
| St-4 | Growth inhibition assay | Growth inhibition of human St-4 cells by sulforhodamine B colorimetric method, GI50 = 0.022 μM. | 23313638 | ||
| HCT15 | Growth inhibition assay | Growth inhibition of human HCT15 cells by sulforhodamine B colorimetric method, GI50 = 0.45 μM. | 23313638 | ||
| NCI-H522 | Cytotoxicity assay | Cytotoxicity against human NCI-H522 cells by SRB assay, GI50 = 0.0018 μM. | 24589486 | ||
| LOXIMVI | Cytotoxicity assay | Cytotoxicity against human LOXIMVI cells by SRB assay, GI50 = 0.0025 μM. | 24589486 | ||
| A549 | Cytotoxicity assay | Cytotoxicity against human A549 cells by SRB assay, GI50 = 0.0026 μM. | 24589486 | ||
| OVCAR5 | Cytotoxicity assay | Cytotoxicity against human OVCAR5 cells by SRB assay, GI50 = 0.0028 μM. | 24589486 | ||
| NCI-H460 | Cytotoxicity assay | Cytotoxicity against human NCI-H460 cells by SRB assay, GI50 = 0.003 μM. | 24589486 | ||
| MKN74 | Cytotoxicity assay | Cytotoxicity against human MKN74 cells by SRB assay, GI50 = 0.003 μM. | 24589486 | ||
| HCC2998 | Cytotoxicity assay | Cytotoxicity against human HCC2998 cells by SRB assay, GI50 = 0.0031 μM. | 24589486 | ||
| HCT116 | Cytotoxicity assay | Cytotoxicity against human HCT116 cells by SRB assay, GI50 = 0.0031 μM. | 24589486 | ||
| MKN1 | Cytotoxicity assay | Cytotoxicity against human MKN1 cells by SRB assay, GI50 = 0.0032 μM. | 24589486 | ||
| HT-29 | Cytotoxicity assay | Cytotoxicity against human HT-29 cells by SRB assay, GI50 = 0.0033 μM. | 24589486 | ||
| SKOV3 | Cytotoxicity assay | Cytotoxicity against human SKOV3 cells by SRB assay, GI50 = 0.0033 μM. | 24589486 | ||
| KM12 | Cytotoxicity assay | Cytotoxicity against human KM12 cells by SRB assay, GI50 = 0.0034 μM. | 24589486 | ||
| SF539 | Cytotoxicity assay | Cytotoxicity against human SF539 cells by SRB assay, GI50 = 0.0036 μM. | 24589486 | ||
| DMS114 | Cytotoxicity assay | Cytotoxicity against human DMS114 cells by SRB assay, GI50 = 0.0036 μM. | 24589486 | ||
| U251 | Cytotoxicity assay | Cytotoxicity against human U251 cells by SRB assay, GI50 = 0.0039 μM. | 24589486 | ||
| SF295 | Cytotoxicity assay | Cytotoxicity against human SF295 cells by SRB assay, GI50 = 0.004 μM. | 24589486 | ||
| MCF7 | Cytotoxicity assay | Cytotoxicity against human MCF7 cells by SRB assay, GI50 = 0.0042 μM. | 24589486 | ||
| OVCAR3 | Cytotoxicity assay | Cytotoxicity against human OVCAR3 cells by SRB assay, GI50 = 0.0046 μM. | 24589486 | ||
| NCI-H23 | Cytotoxicity assay | Cytotoxicity against human NCI-H23 cells by SRB assay, GI50 = 0.0046 μM. | 24589486 | ||
| SF268 | Cytotoxicity assay | Cytotoxicity against human SF268 cells by SRB assay, GI50 = 0.0049 μM. | 24589486 | ||
| MKN7 | Cytotoxicity assay | Cytotoxicity against human MKN7 cells by SRB assay, GI50 = 0.0049 μM. | 24589486 | ||
| OVCAR8 | Cytotoxicity assay | Cytotoxicity against human OVCAR8 cells by SRB assay, GI50 = 0.0055 μM. | 24589486 | ||
| MDA-MB-231 | Cytotoxicity assay | Cytotoxicity against human MDA-MB-231 cells by SRB assay, GI50 = 0.0055 μM. | 24589486 | ||
| DMS273 | Cytotoxicity assay | Cytotoxicity against human DMS273 cells by SRB assay, GI50 = 0.0058 μM. | 24589486 | ||
| DU145 | Cytotoxicity assay | Cytotoxicity against human DU145 cells by SRB assay, GI50 = 0.006 μM. | 24589486 | ||
| RXF631L | Cytotoxicity assay | Cytotoxicity against human RXF631L cells by SRB assay, GI50 = 0.0066 μM. | 24589486 | ||
| HBC4 | Cytotoxicity assay | Cytotoxicity against human HBC4 cells by SRB assay, GI50 = 0.0069 μM. | 24589486 | ||
| SNB75 | Cytotoxicity assay | Cytotoxicity against human SNB75 cells by SRB assay, GI50 = 0.0072 μM. | 24589486 | ||
| BSY1 | Cytotoxicity assay | Cytotoxicity against human BSY1 cells by SRB assay, GI50 = 0.0085 μM. | 24589486 | ||
| NCI-H226 | Cytotoxicity assay | Cytotoxicity against human NCI-H226 cells by SRB assay, GI50 = 0.0089 μM. | 24589486 | ||
| SNB78 | Cytotoxicity assay | Cytotoxicity against human SNB78 cells by SRB assay, GI50 = 0.0096 μM. | 24589486 | ||
| HBC5 | Cytotoxicity assay | Cytotoxicity against human HBC5 cells by SRB assay, GI50 = 0.013 μM. | 24589486 | ||
| MKN45 | Cytotoxicity assay | Cytotoxicity against human MKN45 cells by SRB assay, GI50 = 0.014 μM. | 24589486 | ||
| MKN28 | Cytotoxicity assay | Cytotoxicity against human MKN28 cells by SRB assay, GI50 = 0.017 μM. | 24589486 | ||
| PC3 | Cytotoxicity assay | Cytotoxicity against human PC3 cells by SRB assay, GI50 = 0.018 μM. | 24589486 | ||
| ACHN | Cytotoxicity assay | Cytotoxicity against human ACHN cells by SRB assay, GI50 = 0.02 μM. | 24589486 | ||
| OVCAR4 | Cytotoxicity assay | Cytotoxicity against human OVCAR4 cells by SRB assay, GI50 = 0.02 μM. | 24589486 | ||
| St-4 | Cytotoxicity assay | Cytotoxicity against human St-4 cells by SRB assay, GI50 = 0.022 μM. | 24589486 | ||
| HCT15 | Cytotoxicity assay | Cytotoxicity against human HCT15 cells by SRB assay, GI50 = 0.45 μM. | 24589486 | ||
| 他の多くの細胞株試験データをご覧になる場合はこちらをクリックして下さい | |||||
生物活性
| 製品説明 | ロミデプシン (Romidepsin (FK228, Depsipeptide, FR 901228, NSC 630176)) は強力な HDAC1 および HDAC2 阻害剤 (無細胞アッセイで IC50 = 36 nM/47 nM) です。 ロミデプシン (FK228/Depsipeptide) は、神経芽腫腫瘍細胞 (neuroblastoma tumor cells) の増殖を制御し、アポトーシス (apoptosis) を誘導します。 | ||||
|---|---|---|---|---|---|
| 特性 | More effective than other classical HDAC inhibitors such as TSA, TPX, and butyrate. | ||||
| Targets |
|
| In Vitro | ||||
| In vitro | Unlike TSA, the active form redFK of Romidepsin strongly inhibits HDAC1 and HDAC2 with IC50 of 1.6 nM and 3.9 nM, respectively, but is relatively weak in inhibiting HDAC4 and HDAC6 with IC50 25 nM and 790 nM, respectively. This compound is 17-23 times weaker than redFK in inhibiting these HDACs with IC50 of 36 nM, 47 nM, 510 nM, and 14 μM, respectively. Treatment with this chemical in HeLa cells induces histone acetylation and p21 expression with EC50 of 3.0 nM, more strongly than redFK with EC50 of 11 nM due to the instability of redFK. [1] In addition to G2/M arrest, this compound causes cyclin D1 downregulation and a p53-independent p21 induction, leading to inhibition of CDK and dephosphorylation of Rb resulting in growth arrest in the early G1 phase. [2] It is 100 times more potent than TSA and 1,000,000 times more potent than butyrate in inhibiting the proliferation of the A549 cells. [3] This agent inhibits the growth of U-937, K562, and CCRF-CEM cells with IC50 of 5.92 nM, 8.36 nM, and 6.95 nM, respectively. [5] It promotes apoptosis in chronic lymphocytic leukemia (CLL) cells at a concentration corresponding to that at which H3 and H4 acetylation and HDAC inhibition occurs, selectively involving activation of caspase 8 and effector caspase 3, as well as down-regulation of c-FLIP protein. [6] In 11 of 13 (85%) renal cell carcinoma cell lines and in 16 of 37 (43%) other cancer cell lines, this compound up-regulates tumor death receptors, and potentiates natural killer (NK)-mediated tumor killing. [7] It exhibits concentration-dependent cytotoxicity against a panel of mantle cell lymphoma (MCL) cell lines. [9] | |||
|---|---|---|---|---|
| Kinase Assay | HDAC-inhibitory activity | |||
| For the enzyme assay, 10 μL of [3H]acetyl-labeled histones (25,000 cpm/10 μg) are added to 90 μL of the HDAC enzyme fraction extracted from 293T cells overexpressing HDAC1 or HDAC2 in the presence of increasing concentrations of Romidepsin, and the mixture is incubated at 37 °C for 15 minutes. The enzyme reaction is linear for at least 1 hour. The reaction is stopped by the addition of 10 μL of concentrated HCl. The released [3H]acetic acid is extracted with 1 mL of ethylacetate, and 0.9 mL of the solvent layer is taken into 5 mL of aqueous counting scintillant II solution for determination of radioactivity. The IC50 values are determined from at least three independent dose-response curves. | ||||
| 細胞実験 | 細胞株 | HL60, Jurkat, A549, and MCF-7 | ||
| 濃度 | Dissolved in DMSO, final concentrations ~10 μM | |||
| 反応時間 | 72 hours | |||
| 実験の流れ | Cells are exposed to various concentrations of Romidepsin for 72 hours in 96-well plates. 20 μL of 5 mg/mL MTT solution in PBS is added to each well for 4 hours. After removal of the medium, 170 μL of DMSO is added to each well to dissolve the formazan crystals. The absorbance at 540 nm is determined. In addition, cells are incubated with trypan blue, and the numbers of blue (dead) cells and transparent (live) cells are counted in a hemocytometer. For cell cycle analysis, cells are incubated for 30 minutes in propidium iodide staining solution containing 0.05 mg/mL propidium iodide, 1 mM EDTA, 0.1% Triton X-100, and 1 mg/mL RNase A in PBS. The suspension is then passed through a nylon mesh filter and analyzed on a Becton Dickinson FACScan. |
|||
| 実験結果図 | Methods | Biomarkers | 結果図 | PMID |
| Western blot | p21 / Cyclin D1 AcH3(K9) / Ac-α-Tubulin(K40) / Ac-NFκB2(K310) p65 / 3MeH3(K27) HDAC3 / HDAC4 / HDAC6 / HDAC2 γH2AX / PARP1 / Cleaved caspase3 / BAK / p21(waf1/cip1) / XIAP Cyclin E1 / BRCA1 / E2F1 / Cleaved PARP / H3Ac pAKT(S473) / pAKT(T308) / AKT |

|
19682393 | |
| Growth inhibition assay | Cell viability |
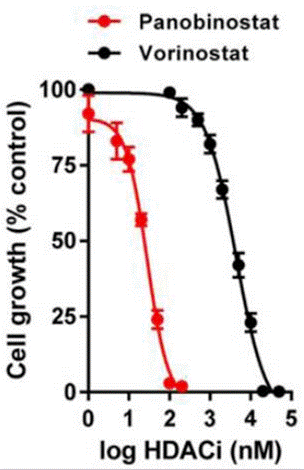
|
27444036 | |
| Immunofluorescence | SS18/TLE1 Cleaved caspase-3 |
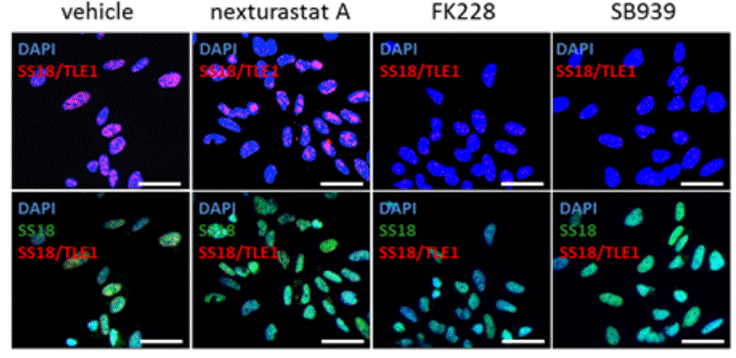
|
27120803 | |
| In Vivo | ||
| In Vivo | Romidepsin treatment potently inhibits the neovascularization of chick embryo and that of adult mice in the Matrigel plug assay. [4]Administration of this compound at 0.1-1 mg/kg twice a week significantly prolongs the survival of mice bearing U-937 lymphoma, with median survival times of 30.5 (0.56 mg/kg) and 33 days (0.32 mg/kg), respectively (vs. 20 days in control mice). [5] | |
|---|---|---|
| 動物実験 | 動物モデル | Male scid mice inoculated i.p. with U-937 cells |
| 投与量 | ~1 mg/kg once or twice a week | |
| 投与経路 | Treated i.p. | |
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT03770000 | Completed | T Cell Lymphoma |
Rhizen Pharmaceuticals SA |
March 12 2019 | Phase 1|Phase 2 |
| NCT02616965 | Active not recruiting | Cutaneous T-cell Lymphoma (CTCL) |
Fox Chase Cancer Center|Seagen Inc.|Celgene Corporation |
February 22 2017 | Phase 1 |
| NCT02616874 | Completed | HIV |
IrsiCaixa|Germans Trias i Pujol Hospital|Fundación FLS de Lucha Contra el Sida las Enfermedades Infecciosas y la Promoción de la Salud y la Ciencia|Hospital Clinic of Barcelona|Hospital de Sant Pau|HIVACAT|University of Oxford|BCN Checkpoint |
February 2016 | Phase 1 |
|
化学情報
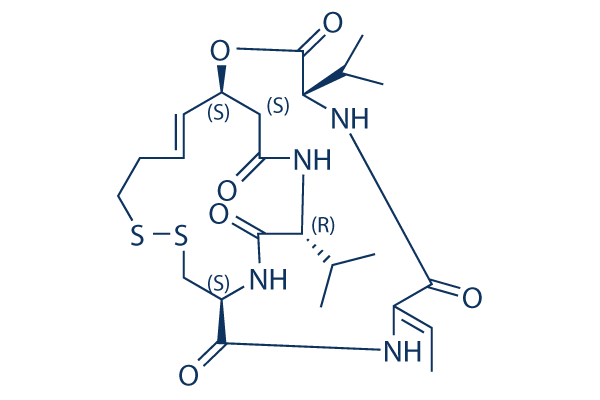
| 分子量 | 540.7 | 化学式 | C24H36N4O6S2 |
| CAS No. | 128517-07-7 | SDF | -- |
| Smiles | CC=C1C(=O)NC(C(=O)OC2CC(=O)NC(C(=O)NC(CSSCCC=C2)C(=O)N1)C(C)C)C(C)C | ||
| 保管 | |||
|
In vitro |
DMSO : 100 mg/mL ( (184.94 mM); 吸湿したDMSOは溶解度を減少させます。新しいDMSOをご使用ください。) Water : Insoluble Ethanol : Insoluble |
モル濃度計算器 |
|
in vivo Add solvents to the product individually and in order. |
投与溶液組成計算機 | |||||
実験計算
投与溶液組成計算機(クリア溶液)
ステップ1:実験データを入力してください。(実験操作によるロスを考慮し、動物数を1匹分多くして計算・調製することを推奨します)
mg/kg
g
μL
匹
ステップ2:投与溶媒の組成を入力してください。(ロット毎に適した溶解組成が異なる場合があります。詳細については弊社までお問い合わせください)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
計算結果:
投与溶媒濃度: mg/ml;
DMSOストック溶液調製方法: mg 試薬を μL DMSOに溶解する(濃度 mg/mL, 注:濃度が当該ロットのDMSO溶解度を超える場合はご連絡ください。 )
投与溶媒調製方法:Take μL DMSOストック溶液に μL PEG300,を加え、完全溶解後μL Tween 80,を加えて完全溶解させた後 μL ddH2O,を加え完全に溶解させます。
投与溶媒調製方法:μL DMSOストック溶液に μL Corn oil,を加え、完全溶解。
注意:1.ストック溶液に沈殿、混濁などがないことをご確認ください;
2.順番通りに溶剤を加えてください。次のステップに進む前に溶液に沈殿、混濁などがないことを確認してから加えてください。ボルテックス、ソニケーション、水浴加熱など物理的な方法で溶解を早めることは可能です。
技術サポート
ストックの作り方、阻害剤の保管方法、細胞実験や動物実験の際に注意すべき点など、製品を取扱う時に問い合わせが多かった質問に対しては取扱説明書でお答えしています。
他に質問がある場合は、お気軽にお問い合わせください。
* 必須