- 阻害剤
- 研究分野別
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- 化合物ライブラリー
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-II
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at info@selleck.co.jp to customize your library.
You could select:
- 抗体
- 新製品
- お問い合わせ
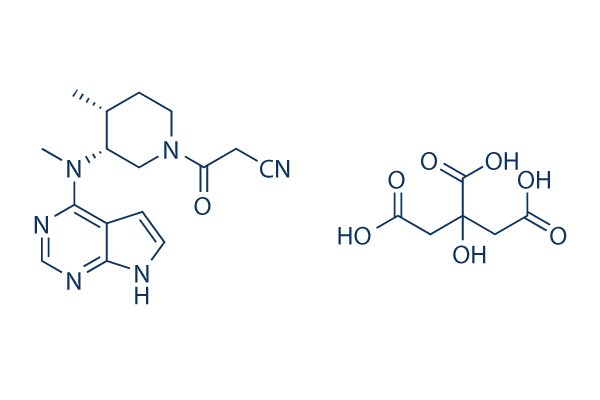
CP-690550 (Tofacitinib) Citrate
別名:Tasocitinib Citrate,CP-690550
トファシチニブクエン酸塩 (Tofacitinib citrate (CP-690550、タソシチニブ)) は JAK の新規阻害剤です。IC50は JAK3, JAK2, JAK1 に対してそれぞれ 1 nM, 20 nM, 112 nM です。 トファシチニブクエン酸塩は抗感染作用を持ちます。
CAS No. 540737-29-9
文献中Selleckの製品使用例(191)
製品安全説明書
現在のバッチを見る:
純度:
99.99%
99.99
CP-690550 (Tofacitinib) Citrate関連製品
シグナル伝達経路
JAK阻害剤の選択性比較
Cell Data
| Cell Lines | Assay Type | Concentration | Incubation Time | 活性情報 | PMID |
|---|---|---|---|---|---|
| NK | Immunosuppressive assay | 1 to 5 mg/kg | 4 weeks | Immunosuppressive activity in naive cynomolgus monkey assessed as effect on CD3-CD16+ NK cells at 1 to 5 mg/kg, po for 4 weeks | 14593182 |
| T-cells | Function assay | 5 to 500 nM | 1 hr | Inhibition of IL2-induced Stat5 phosphorylation in human CD4+ T cells at 5 to 500 nM after 1 hr by Western blot | 19053756 |
| SUM149PT | Function assay | 3 uM | 16 hrs | Induction of PTPN6 in human SUM149PT cells assessed as inhibition of STAT3 phosphorylation at 3 uM after 16 hrs by Western blotting analysis in presence of pervanadate | 24978112 |
| Hs578T | Function assay | 3 uM | 16 hrs | Induction of PTPN6 in human Hs578T cells assessed as inhibition of STAT3 phosphorylation at 3 uM after 16 hrs by Western blotting analysis in presence of pervanadate | 24978112 |
| T-cells | Function assay | 50 to 300 nM | 1 hr | Inhibition of JAK1/JAK3 in human CD4 positive T cells assessed as inhibition of IL4-stimulated STAT6 phosphorylation at 50 to 300 nM preincubated for 1 hr followed by IL-4 stimulation measured after 30 mins by immunoblot analysis | 29852068 |
| T-cells | Function assay | 50 to 300 nM | 1 hr | Inhibition of JAK1/JAK3 in human CD4 positive T cells assessed as inhibition of IL2-stimulated STAT5 phosphorylation at Tyr-695 residue at 50 to 300 nM preincubated for 1 hr followed by IL-2 stimulation measured after 30 mins by immunoblot analysis | 29852068 |
| T-cells | Function assay | >300 nM | 1 hr | Inhibition of JAK1/JAK2/TYK2 in human CD4 positive T cells assessed as inhibition of IL6-stimulated STAT3 phosphorylation at >300 nM preincubated for 1 hr followed by IL-6 stimulation measured after 30 mins by immunoblot analysis | 29852068 |
| TALL-1 | Function assay | 1 uM | 3 hrs | Inhibition of JAK3 in human TALL-1 cells assessed as inhibition of IL-2 induced STAT5 phosphorylation at 1 uM preincubated for 3 hrs followed by IL-2 induction measured after 30 mins by immunoblotting | 26258521 |
| BA/F3 | Function assay | 300 nM | 3 hrs | Inhibition of JAK3 (unknown origin) expressed in mouse BA/F3 cells assessed as reduction of STAT5 phosphorylation at 300 nM after 3 hrs by immunoblotting analysis | 26258521 |
| OCL-AML5 | Function assay | 1 uM | 3 hrs | Inhibition of JAK2 in human OCL-AML5 cells assessed as inhibition of GM-CSF induced STAT5 phosphorylation at 1 uM preincubated for 3 hrs followed by GM-CSF induction measured after 30 mins by immunoblotting | 26258521 |
| BA/F3 | Function assay | 1 uM | 3 hrs | Inhibition of JAK3 (unknown origin) expressed in mouse BA/F3 cells at 1 uM preincubated for 3 hrs followed by pulldown with streptavidin beads by immunoblotting analysis | 26258521 |
| Huh7 | Function assay | 10 uM | 30 mins | Inhibition of Tyk2 in human Huh7 cells assessed as reduction of IFNalpha5-induced STAT3 phosphorylation at 10 uM pre-incubated for 30 mins before IFNalpha5 stimulation for 30 mins mins by immunoblotting | 26231159 |
| Huh7 | Function assay | 10 uM | 30 mins | Inhibition of Tyk2 in human Huh7 cells assessed as reduction of basal level STAT3 phosphorylation at 10 uM after 30 mins by immunoblotting | 26231159 |
| Huh7 | Function assay | 1 uM | 30 mins | Inhibition of Tyk2 in human Huh7 cells assessed as reduction of IFNalpha5-induced STAT3 phosphorylation at 1 uM pre-incubated for 30 mins before IFNalpha5 stimulation for 30 mins mins by immunoblotting | 26231159 |
| YT | Function assay | 30 ng/ml | Inhibition of IL2-induced JAK3 phosphorylation in human YT cells at 30 ng/ml by immunoblotting analysis | 14593182 | |
| YT | Function assay | 30 ng/ml | Inhibition of IL2-induced STAT5A phosphorylation in human YT cells at 30 ng/ml by immunoblotting analysis | 14593182 | |
| YT | Function assay | 30 ng/ml | Inhibition of IL2-induced STAT5B phosphorylation in human YT cells at 30 ng/ml by immunoblotting analysis | 14593182 | |
| TF1 | Function assay | 20 mins | Inhibition of JAK1 in human TF1 cells assessed as inhibition of IL6-induced STAT3 phosphorylation incubated for 20 mins followed by IL6 challenge for 30 mins in presence of whole blood, EC50 = 0.043 μM. | 23659214 | |
| Ba/F3 | Function assay | 60 mins | Inhibition of TEL-fused JAK1 expressed in Ba/F3 cells assessed as inhibition of STAT5 phosphorylation after 60 mins by AlphaScreen assay, IC50 = 0.026 μM. | 22087750 | |
| T-cells | Function assay | 72 hrs | Inhibition of IL2-induced proliferation of human T cells assessed as [3H]thymidine incorporation after 72 hrs by scintillation counting, IC50 = 0.011 μM. | 14593182 | |
| SF21 | Function assay | 10 mins | Inhibition of JAK2 (unknown origin) expressed in SF21 cells using Biotin-KAIETDKEYYTVKD as substrate and [33Pgamma]ATP incubated for 10 mins prior to substrate addition measured after 30 mins by Topcount analysis, IC50 = 0.004 μM. | 23541670 | |
| Sf9 | Function assay | 90 mins | Inhibition of human recombinant N-terminal GST-tagged JAK2 expressed in Sf9 cells using Biotin-LC-EQEDEPEGDYFEWLE as substrate after 90 mins by TR-FRET assay, IC50 = 0.004 μM. | 22087750 | |
| Sf9 | Function assay | 90 mins | Inhibition of human recombinant N-terminal GST-tagged JAK1 expressed in Sf9 cells using Biotin-LC-EQEDEPEGDYFEWLE as substrate after 90 mins by TR-FRET assay, IC50 = 0.003 μM. | 22087750 | |
| Sf9 | Function assay | 30 mins | Inhibition of human GST-fused JAK2 catalytic domain expressed in baculovirus-infected Sf9 cells using polyglutamic acid-tyrosine as substrate after 30 mins by ELISA, Ki = 0.0007 μM. | 23668484 | |
| Sf9 | Function assay | 30 mins | Inhibition of human GST-fused JAK1 catalytic domain expressed in baculovirus-infected Sf9 cells using polyglutamic acid-tyrosine as substrate after 30 mins by ELISA, Ki = 0.0007 μM. | 23668484 | |
| Sf9 | Function assay | 90 mins | Inhibition of human recombinant N-terminal GST-tagged JAK3 expressed in Sf9 cells using Biotin-LC-EQEDEPEGDYFEWLE as substrate after 90 mins by TR-FRET assay, IC50 = 0.0006 μM. | 22087750 | |
| Sf9 | Function assay | 30 mins | Inhibition of human GST-fused JAK3 catalytic domain expressed in baculovirus-infected Sf9 cells using polyglutamic acid-tyrosine as substrate after 30 mins by ELISA, Ki = 0.0004 μM. | 23668484 | |
| Sf9 | Function assay | 90 mins | Inhibition of human recombinant N-terminal GST-tagged TYK2 expressed in Sf9 cells using Biotin-LC-EQEDEPEGDYFEWLE as substrate after 90 mins by TR-FRET assay, IC50 = 0.052 μM. | 22087750 | |
| TF1 | Function assay | 20 mins | Inhibition of JAK1 in human TF1 cells assessed as inhibition of IL6-induced STAT3 phosphorylation incubated for 20 mins prior to IL6-induction measured after 30 to 45 mins, EC50 = 0.053 μM. | 22698084 | |
| TF1 | Function assay | 20 mins | Inhibition of JAK1 in human TF1 cells assessed as inhibition of IL6-induced STAT3 phosphorylation incubated for 20 mins followed by IL6 challenge for 30 mins, EC50 = 0.053 μM. | 23659214 | |
| Ba/F3 | Function assay | 60 mins | Inhibition of TEL-fused JAK3 expressed in Ba/F3 cells assessed as inhibition of STAT5 phosphorylation after 60 mins by AlphaScreen assay, IC50 = 0.054 μM. | 22087750 | |
| TF1 | Function assay | 20 mins | Inhibition of JAK2 in human TF1 cells assessed as inhibition of EPO-induced STAT5 phosphorylation incubated for 20 mins prior to EPO-induction measured after 30 to 45 mins, EC50 = 0.093 μM. | 22698084 | |
| TF1 | Function assay | 20 mins | Inhibition of JAK2 in human TF1 cells assessed as inhibition of EPO-induced STAT5 phosphorylation incubated for 20 mins followed by IL6 challenge for 30 mins, EC50 = 0.093 μM. | 23659214 | |
| TF1 | Function assay | 2 hrs | Inhibition of JAK2 in GMCSF-stimulated human TF1 cells assessed as suppression of STAT5 phosphorylation preincubated for 2 hrs followed by GMCSF stimulation for 50 mins by FACS reader analysis, IC50 = 0.095 μM. | 27130359 | |
| CTLL-2 | Antiproliferative assay | 72 hrs | Antiproliferative activity against IL-2-stimulated mouse CTLL-2 cells expressing JAK1/JAK3 after 72 hrs by alamar blue assay, IC50 = 0.132 μM. | 19762238 | |
| DND/L12 | Function assay | 30 mins | Inhibition of JAK3 in human DND/L12 cells after 30 mins by luciferase assay in presence of human serum albumin, IC90 = 0.16 μM. | 14593182 | |
| Ba/F3 | Function assay | 60 mins | Inhibition of TEL-fused JAK2 expressed in Ba/F3 cells assessed as inhibition of STAT5 phosphorylation after 60 mins by AlphaScreen assay, IC50 = 0.265 μM. | 22087750 | |
| CD34+ | Function assay | 45 mins | Inhibition of JAK2 homodimer in human CD34+ cells spiked into human whole blood assessed as inhibition of EPO-induced STAT-5 phosphorylation preincubated for 45 mins followed by EPO addition measured after 15 mins by FACS analysis, IC50 = 0.302 μM. | 24417533 | |
| BA/F3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against mouse BA/F3 cells expressing TEL-JAK3 after 72 hrs by cell titer glo assay | 26258521 | |
| TF1 | Function assay | Inhibition of JAK1 in human TF1 cells assessed as suppression of IL6-stimulated STAT3 phosphorylation by AlphaScreen assay, INH = 0.045 μM. | 26372653 | ||
| PBMC | Function assay | Inhibition IL-7-indcued STAT5 phosphorylation in human PBMC cells by flow cytometry, IC50 = 0.039 μM. | 26927423 | ||
| T-cells | Function assay | Inhibition of JAK3/1 in human T cells expressing CD3 assessed as inhibition of IL2-stimulated STAT5a phosphorylation, IC50 = 0.028 μM. | 23540648 | ||
| MO7 | Function assay | Inhibition of Jak3-mediated IL15-induced Stat5 phosphorylation in human MO7 cells by cell-based assay, IC50 = 0.024 μM. | 21155605 | ||
| insect | Function assay | Inhibition of GST-tagged Jak2 expressed in insect cells using 20 uM ATP, IC50 = 0.012 μM. | 21155605 | ||
| insect cells | Function assay | Inhibition of GST-tagged Jak3 expressed in insect cells using 18 uM ATP, IC50 = 0.008 μM. | 21155605 | ||
| insect cells | Function assay | Inhibition of GST-tagged Jak1 expressed in insect cells using 70 uM ATP, IC50 = 0.0061 μM. | 21155605 | ||
| Sf9 | Function assay | Inhibition of GST-tagged human JAK3 catalytic domain expressed in Sf9 cells by ELISA, IC50 = 0.0033 μM. | 21105711 | ||
| Sf9 | Function assay | Inhibition of GST-tagged human JAK3 catalytic domain expressed in Sf9 cells by ELISA, IC50 = 0.001 μM. | 21105711 | ||
| Sf9 | Function assay | Inhibition of human recombinant GST-tagged JH1 domain (785 to1125) of JAK3 expressed in insect Sf9 cells by ELISA, IC50 = 0.001 μM. | 14593182 | ||
| CTLL | Function assay | Inhibition of Jak3-mediated IL2-induced Stat5 phosphorylation in mouse CTLL cells by cell-based assay, IC50 = 0.048 μM. | 21155605 | ||
| TF1 | Function assay | Inhibition of JAK1 in human TF1 cells assessed as inhibition of IL2-induced STAT3 phosphorylation, EC50 = 0.053 μM. | 22591402 | ||
| T-cells | Function assay | Inhibition of allogenic cells-stimulated proliferation in monkey T cells by mixed lymphocyte reaction method, IC50 = 0.057 μM. | 14593182 | ||
| CD34+ | Function assay | Inhibition of JAK2 in human CD34+ cells assessed as inhibition of EPO-mediated cell proliferation, IC50 = 0.071 μM. | 26927423 | ||
| TF1 | Function assay | Inhibition of IL4-induced proliferation of TF1 cells, IC50 = 0.08 μM. | 16934457 | ||
| T-cells | Function assay | Inhibition of allogenic cells-stimulated proliferation in human T cells by mixed lymphocyte reaction method, IC50 = 0.087 μM. | 14593182 | ||
| PBMC | Function assay | Inhibition of JAK1 in human PBMC cells assessed as inhibition of IL-6-induced MCP1 secretion, IC50 = 0.095 μM. | 26927423 | ||
| TF1 | Function assay | Inhibition of JAK2 in EPO-stimulated human TF1 cells expressing stably integrated beta-lactamase reporter gene under control of STAT5 response elements in interferon regulatory factor 1 gene promoter by fluorescence assay, IC50 = 0.107 μM. | 29156136 | ||
| Sf9 | Function assay | Inhibition of GST-tagged human JAK1 catalytic domain expressed in Sf9 cells by ELISA, IC50 = 0.11 μM. | 21105711 | ||
| T-cells | Function assay | Inhibition of allogenic cells-stimulated proliferation in mouse T cells by mixed lymphocyte reaction method, IC50 = 0.115 μM. | 14593182 | ||
| ME180 | Function assay | Inhibition of JAK1 in IL6-stimulated human ME180 cells expressing stably integrated beta-lactamase reporter gene under control of sis-inducible element by fluorescence assay, IC50 = 0.124 μM. | 29156136 | ||
| HT2 | Function assay | Inhibition of JAK3 in mouse HT2 cells assessed as suppression of cell growth, IC50 = 0.156 μM. | 27771180 | ||
| T-cells | Function assay | Inhibition of JAK2/1 in human T cells expressing CD3 assessed as inhibition of IFNgamma-stimulated STAT1 phosphorylation, IC50 = 0.17 μM. | 23540648 | ||
| insect cells | Function assay | Inhibition of GST-tagged Tyk2 expressed in insect cells using 35 uM ATP, IC50 = 0.176 μM. | 21155605 | ||
| TF1 | Function assay | Inhibition of JAK2 in human TF1 cells assessed as suppression of cell growth, IC50 = 0.2751 μM. | 27771180 | ||
| A2780 | Function assay | Inhibition of cdk-mediated NPM phosphorylation at thr199 in human A2780 cells | 14593182 | ||
| A2780 | Function assay | Inhibition of cdk-mediated NPM phosphorylation at thr199 in human A2780 cells | 21105711 | ||
| A2780 | Function assay | Inhibition of cdk-mediated NPM phosphorylation at thr199 in human A2780 cells | 19762238 | ||
| A2780 | Function assay | Inhibition of cdk-mediated NPM phosphorylation at thr199 in human A2780 cells | 30460842 | ||
| A2780 | Function assay | Inhibition of cdk-mediated NPM phosphorylation at thr199 in human A2780 cells | 23659214 | ||
| A2780 | Function assay | Inhibition of cdk-mediated NPM phosphorylation at thr199 in human A2780 cells | 16934457 | ||
| A2780 | Function assay | Inhibition of cdk-mediated NPM phosphorylation at thr199 in human A2780 cells | 26372653 | ||
| A2780 | Function assay | Inhibition of cdk-mediated NPM phosphorylation at thr199 in human A2780 cells | 22087750 | ||
| A2780 | Function assay | Inhibition of cdk-mediated NPM phosphorylation at thr199 in human A2780 cells | 30139575 | ||
| A2780 | Function assay | Inhibition of cdk-mediated NPM phosphorylation at thr199 in human A2780 cells | 27771180 | ||
| 他の多くの細胞株試験データをご覧になる場合はこちらをクリックして下さい | |||||
生物活性
| 製品説明 | トファシチニブクエン酸塩 (Tofacitinib citrate (CP-690550、タソシチニブ)) は JAK の新規阻害剤です。IC50は JAK3, JAK2, JAK1 に対してそれぞれ 1 nM, 20 nM, 112 nM です。 トファシチニブクエン酸塩は抗感染作用を持ちます。 | ||||
|---|---|---|---|---|---|
| Targets |
|
| In Vitro | ||||
| In vitro | Tofacitinib citrate inhibits IL-2-mediated human T cell blast proliferation and IL-15-induced CD69 expression with IC50 of 11 nM and 48 nM, respectively. Tofacitinib citrate prevents mixed lymphocyte reaction with IC50 of 87 nM. Tofacitinib citrate treatment of murine factor-dependent cell Patersen–erythropoietin receptor (FDCP-EpoR) cells harboring human wild-type or V617F JAK2 leads to prevention of cell proliferation with IC50 of 2.1 µM and 0.25 µM, respectively. Tofacitinib citrate inhibits interleukin-6-induced phosphorylation of STAT1 and STAT3 with IC50 of 23 nM and 77 nM, respectively. Moreover, Tofacitinib citrate generates a significant pro-apoptotic effect on murine FDCP-EpoR cells carrying JAK2VV617F, whereas a lesser effect is observed for cells carrying wild-type JAK2. This activity is coupled with the inhibition of phosphorylation of the key JAK2V617F-dependent downstream signaling effectors signal transducer and activator of transcription (STAT)3, STAT5, and v-akt murine thymoma viral oncogene homolog (AKT). Additionally, Tofacitinib citrate prevents IL-15-induced CD69 expression in human and cynomolgus monkey NK and CD8+ T cells in vitro. | |||
|---|---|---|---|---|
| Kinase Assay | Enzyme assays | |||
| The JAK1, JAK2, and JAK3 kinase assays utilize a protein expressed in baculovirus-infected SF9 cells (a fusion protein of GST and the catalytic domain of human JAK enzyme) purified by affinity chromatography on glutathione−Sepharose. The substrate for the reaction is polyglutamic acid-tyrosine [PGT (4:1)], coated onto Nunc Maxi Sorp plates at 100 μg/mL overnight at 37 °C. The plates are washed three times, and JAK enzyme is added to the wells, which contained 100 μL of kinase buffer (50 mM HEPES, pH 7.3, 125 mM NaCl, 24 mM MgCl2) + ATP + 1 mM sodium orthovanadate). For Tofacitinib citrate, it is also added for kinase assay at different doses. After incubation at room temperature for 30 min, the plates are washed three times. The level of phosphorylated tyrosine in a given well is determined by standard ELISA assay utilizing an anti-phosphotyrosine antibody. | ||||
| 細胞実験 | 細胞株 | FDCP-EpoR JAK2WT and JAK2V617F cell lines | ||
| 濃度 | 0-4 μM | |||
| 反応時間 | 72 hours | |||
| 実験の流れ | Determination of growth inhibition by Tofacitinib citrate is performed using identical culture conditions for both FDCP-EpoR JAK2WT and JAK2V617F cell lines. Briefly, 1 × 105 cells/mL are cultured in 96-well flat-bottom plates at 37 °C in a humidified 5% CO2 atmosphere using RPMI 1640 supplemented with 1.25% FCS, and 5% WEHI supernatant. Decreased FCS concentration is necessary to prevent binding between Tofacitinib citrate and serum proteins. Growth inhibition assays are terminated by addition of 20 μL CellTiter96 One Solution Reagent. Flat-bottom plates are incubated for an additional 3 hours for MTT assay. Absorbance is determined at 595 nm on a BioTek Synergy-HT microplate reader. Results are the average standard deviation of three independent determinations. |
|||
| 実験結果図 | Methods | Biomarkers | 結果図 | PMID |
| Western blot | JAK3 / p-JAK3 / STAT3 LMP1 / EBNA1 / BZLF1 JAK3 / STAT5 / p-STAT5 |
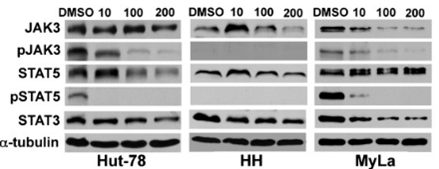
|
26082451 | |
| Growth inhibition assay | Cell number |
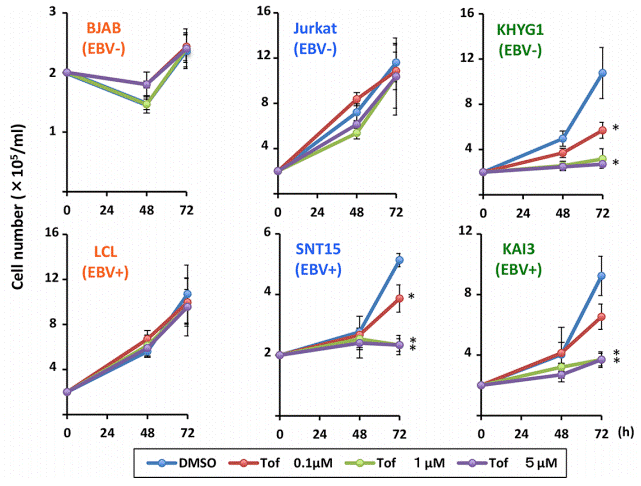
|
27732937 | |
| In Vivo | ||
| In Vivo | Tofacitinib citrate decrease a delayed-type hyper-sensitivity response and extended cardiac allograft survival in murine models. Furthermore, Tofacitinib citrate treatment of ex-vivo-expanded erythroid progenitors from JAK2V617F-positive PV patients results in specific, antiproliferative (IC50 = 0.2 μM) and pro-apoptotic activity. In contrast, expanded progenitors from healthy controls are less sensitive to Tofacitinib citrate in proliferation (IC50 > 1.0 μM), and apoptosis assays. During 2 weeks of Tofacitinib citrate dosing at 10 and 30 mg/kg/d, a significant, time-dependent decrease in NK cell numbers relative to vehicle treatment is observed. Effector memory CD8+ cell numbers in the Tofacitinib citrate-treated group are 55% less than those observed in animals treated with vehicle. | |
|---|---|---|
| 動物実験 | 動物モデル | Mauritius-origin adult cynomolgus monkeys |
| 投与量 | 10, 30 mg/kg/d | |
| 投与経路 | Oral gavage | |
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06202560 | Enrolling by invitation | Frontal Fibrosing Alopecia|Lichen Planopilaris |
Institute of Dermatology Thailand |
November 29 2023 | Not Applicable |
| NCT05487703 | Completed | Arthritis Rheumatoid |
Pfizer |
November 14 2022 | -- |
| NCT05082428 | Completed | Ulcerative Colitis |
Pfizer |
May 30 2022 | -- |
| NCT05728008 | Completed | Ulcerative Colitis |
IRCCS San Raffaele |
April 5 2022 | -- |
| NCT04768504 | Recruiting | Immune-Mediated Colitis |
Khashayar Esfahani|Sir Mortimer B. Davis - Jewish General Hospital |
March 22 2022 | Phase 2 |
|
化学情報
| 分子量 | 504.49 | 化学式 | C16H20N6O.C6H8O7 |
| CAS No. | 540737-29-9 | SDF | Download CP-690550 (Tofacitinib) Citrate SDFをダウンロードする |
| Smiles | CC1CCN(CC1N(C)C2=NC=NC3=C2C=CN3)C(=O)CC#N.C(C(=O)O)C(CC(=O)O)(C(=O)O)O | ||
| 保管 | |||
|
In vitro |
DMSO : 100 mg/mL ( (198.21 mM); 吸湿したDMSOは溶解度を減少させます。新しいDMSOをご使用ください。) Water : Insoluble Ethanol : Insoluble |
モル濃度計算器 |
|
in vivo Add solvents to the product individually and in order. |
投与溶液組成計算機 | |||||
実験計算
投与溶液組成計算機(クリア溶液)
ステップ1:実験データを入力してください。(実験操作によるロスを考慮し、動物数を1匹分多くして計算・調製することを推奨します)
mg/kg
g
μL
匹
ステップ2:投与溶媒の組成を入力してください。(ロット毎に適した溶解組成が異なる場合があります。詳細については弊社までお問い合わせください)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
計算結果:
投与溶媒濃度: mg/ml;
DMSOストック溶液調製方法: mg 試薬を μL DMSOに溶解する(濃度 mg/mL, 注:濃度が当該ロットのDMSO溶解度を超える場合はご連絡ください。 )
投与溶媒調製方法:Take μL DMSOストック溶液に μL PEG300,を加え、完全溶解後μL Tween 80,を加えて完全溶解させた後 μL ddH2O,を加え完全に溶解させます。
投与溶媒調製方法:μL DMSOストック溶液に μL Corn oil,を加え、完全溶解。
注意:1.ストック溶液に沈殿、混濁などがないことをご確認ください;
2.順番通りに溶剤を加えてください。次のステップに進む前に溶液に沈殿、混濁などがないことを確認してから加えてください。ボルテックス、ソニケーション、水浴加熱など物理的な方法で溶解を早めることは可能です。
技術サポート
ストックの作り方、阻害剤の保管方法、細胞実験や動物実験の際に注意すべき点など、製品を取扱う時に問い合わせが多かった質問に対しては取扱説明書でお答えしています。
他に質問がある場合は、お気軽にお問い合わせください。
* 必須
よくある質問(FAQ)
質問1:
What is the difference between the two products (S5001, S2789)?
回答
Tofacitinib (S2789) is the base form of this compound. The biological activity of these two compounds is the same. However, S5001 is better than S2789 for oral gavage.