
- 阻害剤
- 研究分野別
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- 化合物ライブラリー
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-II
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at info@selleck.co.jp to customize your library.
You could select:
- 抗体
- 新製品
- お問い合わせ
Ibrutinib (PCI-32765)
別名:PCI-32765
イブルチニブ (Ibrutinib (PCI-32765)) は、無細胞アッセイで IC50 が 0.5 nM の強力で選択性の高い Brutons チロシンキナーゼ (Brutons tyrosine kinase, Btk) 阻害剤であり、Bmx, CSK, FGR, BRK, HCKに対しても阻害活性を持ちます。 EGFR, Yes, ErbB2, JAK3 に対しては前者より比較的弱い活性を持ちます。イブルチニブは、P13I を含む一連の PROTAC の合成における Btk リガンドとして適用できます。
CAS No. 936563-96-1
文献中Selleckの製品使用例(543)
製品安全説明書
現在のバッチを見る:
純度:
99.98%
99.98
Ibrutinib (PCI-32765)関連製品
Target Protein Ligand阻害剤の選択性比較
Cell Data
| Cell Lines | Assay Type | Concentration | Incubation Time | 活性情報 | PMID |
|---|---|---|---|---|---|
| human Rec1 cells | Function assay | 2.5 μM | 6 h | Inhibition of Lyn phosphorylation in human Rec1 cells at 2.5 uM incubated for 6 hrs by Western blotting method | 25222877 |
| human Ramos cells | Function assay | 1 h | Inhibition of Btk in human Ramos cells assessed as inhibition of PLC-gamma2 phosphorylation at Tyr1217 after 1 hr by Western blot analysis, IC50=14 nM. | 24915291 | |
| Sf9 cells | Function assay | 1 h | Inhibition of LYN-A expressed in Sf9 cells after 60 mins by TR-FRET Assay, IC50=0.2 μM. | 21958547 | |
| human DOHH2 cells | Cytotoxic assay | 72 h | Cytotoxicity against human DOHH2 cells assessed as growth inhibition after 72 hrs by CellTiter-Glo luminescent cell viability assay, IC50=0.41 μM. | 24915291 | |
| human SU-DHL6 cells | Cytotoxic assay | 72 h | Cytotoxicity against human SU-DHL6 cells assessed as growth inhibition after 72 hrs by CellTiter-Glo luminescent cell viability assay, IC50=0.58 μM. | 24915291 | |
| human WSU-NHL cells | Cytotoxic assay | 72 h | Cytotoxicity against human WSU-NHL cells assessed as growth inhibition after 72 hrs by CellTiter-Glo luminescent cell viability assay, IC50=1.09 μM | 24915291 | |
| human Pfeiffer cells | Function assay | 72 h | Cytotoxicity against human Pfeiffer cells assessed as growth inhibition after 72 hrs by CellTiter-Glo luminescent cell viability assay, IC50=2 nM. | 24915291 | |
| Sf9 cells | Function assay | 1 h | Inhibition of human full-length BTK expressed in Sf9 cells using FAM-Srctide peptide as substrate after 60 mins by TR-FRET Assay, IC50=0.5 nM. | 21958547 | |
| Sf9 insect cells | Function assay | 60 mins | IC50 = 0.0003 μM | 29146136 | |
| Sf9 insect cells | Function assay | 60 mins | IC50 = 0.00034 μM | 27912175 | |
| Sf9 insect cells | Function assay | 60 mins | IC50 = 0.00034 μM | 28432946 | |
| Sf9 insect cells | Function assay | 60 mins | IC50 = 0.00034 μM | 27956037 | |
| Sf9 cells | Function assay | 60 mins | IC50 = 0.0004 μM | 30006143 | |
| Sf9 cells | Function assay | 60 mins | IC50 = 0.0005 μM | 21958547 | |
| Ramos cells | Function assay | 1 h | IC50 = 0.0005 μM | 28280261 | |
| Sf9 cells | Function assay | 60 mins | IC50 = 0.001 μM | 30077608 | |
| Pfeiffer cells | Cytotoxicity assay | 72 h | GI50 = 0.002 μM | 24915291 | |
| B cells | Function assay | 1 h | IC50 = 0.0046 μM | 30290988 | |
| Sf9 insect cells | Function assay | 2 to 60 mins | Ki = 0.0048 μM | 28315597 | |
| Ramos cells | Function assay | 1 h | IC50 = 0.0075 μM | 24915291 | |
| Sf9 insect cells | Function assay | 60 mins | IC50 = 0.008 μM | 28315597 | |
| TMD8 cells | Antiproliferative activity assay | 72 h | IC50 = 0.01 μM | 29715023 | |
| CD19+ B cells | Function assay | 1 h | IC50 = 0.012 μM | 29457982 | |
| Sf9 insect cells | Function assay | 60 mins | IC50 = 0.012 μM | 28315597 | |
| Ramos cells | Function assay | 1 h | IC50 = 0.014 μM | 24915291 | |
| Sf9 insect cells | Function assay | 1 h | IC50 = 0.0144 μM | 29715023 | |
| Sf9 insect cells | Function assay | 60 mins | IC50 = 0.0161 μM | 29146136 | |
| HCC827 cells | Antiproliferative activity assay | 72 h | IC50 = 0.039 μM | 28734581 | |
| PC9 cells | Function assay | 72 h | GI50 = 0.05 μM | 28282122 | |
| Sf9 cells | Function assay | 60 mins | IC50 = 0.1 μM | 30006143 | |
| H3255 cells | Function assay | 72 h | GI50 = 0.11 μM | 28282122 | |
| BaF3 cells | Function assay | 72 h | GI50 = 0.12 μM | 26630553 | |
| BAF3 cells | Antiproliferative activity assay | 72 h | GI50 = 0.12 μM | 28956923 | |
| Sf9 insect cells | Function assay | 60 mins | IC50 = 0.123 μM | 28315597 | |
| Sf9 insect cells | Function assay | 60 mins | IC50 = 0.146 μM | 28315597 | |
| BAF3 cells | Function assay | 72 h | GI50 = 0.16 μM | 28282122 | |
| Sf9 cells | Function assay | 60 mins | IC50 = 0.2 μM | 21958547 | |
| MV4-11 cells | Antiproliferative activity assay | 72 h | GI50 = 0.33 μM | 26630553 | |
| MV4-11 cells | Antiproliferative activity assay | 72 h | GI50 = 0.33 μM | 28956923 | |
| DOHH2 cells | Cytotoxicity assay | 72 h | GI50 = 0.41 μM | 24915291 | |
| HCC827 cells | Antiproliferative activity assay | 96 h | EC50 = 0.45 μM | 28853575 | |
| SU-DHL6 cells | Cytotoxicity assay | 72 h | GI50 = 0.58 μM | 24915291 | |
| NCI-H1975 cells | Antiproliferative activity assay | 96 h | EC50 = 0.64 μM | 28853575 | |
| HEK293T cells | Function assay | 1 h | IC50 = 0.9 μM | 28280261 | |
| Ramos cells | Antiproliferative activity assay | 72 h | IC50 = 0.92 μM | 29715023 | |
| BA/F3 cells | Antiproliferative activity assay | 72 h | IC50 = 1 μM | 26258521 | |
| WSU-NHL cells | Cytotoxicity assay | 72 h | GI50 = 1.09 μM | 24915291 | |
| NCI-H1975 cells | Function assay | 72 h | GI50 = 1.2 μM | 28282122 | |
| NCI-H1975 cells | Antiproliferative activity assay | 72 h | IC50 = 1.27 μM | 28734581 | |
| Raji cells | Antiproliferative activity assay | 48 h | IC50 = 1.49 μM | 29567295 | |
| A431 cells | Antiproliferative activity assay | 96 h | EC50 = 2.38 μM | 28853575 | |
| BAF3 cells | Antiproliferative activity assay | 72 h | GI50 = 2.5 μM | 28956923 | |
| Ramos cells | Antiproliferative activity assay | 72 h | IC50 = 5.14 μM | 30006143 | |
| Ramos cells | Antiproliferative activity assay | 48 h | IC50 = 5.14 μM | 27956037 | |
| Ramos cells | Antiproliferative activity assay | 48 h | IC50 = 5.88 μM | 28432946 | |
| Ramos cells | Antiproliferative activity assay | 72 h | IC50 = 6.62 μM | 29146136 | |
| K562 cells | Antiproliferative activity assay | 48 h | IC50 = 7.5 μM | 30077608 | |
| HL60 cells | Antiproliferative activity assay | 48 h | IC50 = 8 μM | 30077608 | |
| Ramos cells | Antiproliferative activity assay | 48 h | IC50 = 8.11 μM | 27994736 | |
| Ramos cells | Antiproliferative activity assay | 48 h | IC50 = 8.26 μM | 29567295 | |
| BAF3 cells | Cytotoxicity assay | 72 h | GI50 = 10 μM | 26630553 | |
| BAF3 cells | Antiproliferative activity assay | 72 h | GI50 = 10 μM | 28956923 | |
| BAF3 cells | Growth inhibition assay | 72 h | GI50 = 10 μM | 28282122 | |
| NAMALWA cells | Antiproliferative activity assay | 72 h | IC50 = 10.45 μM | 29146136 | |
| Ramos cells | Antiproliferative activity assay | 48 h | IC50 = 12.6 μM | 27912175 | |
| Raji cells | Antiproliferative activity assay | 48 h | IC50 = 14.2 μM | 30077608 | |
| Raji cells | Antiproliferative activity assay | 48 h | IC50 = 15.2 μM | 27994736 | |
| MIAPaCa2 cells | Cytotoxicity assay | 3 days | IC50 = 16.6 μM | 27077228 | |
| HeLa cells | Cytotoxicity assay | 3 days | IC50 = 16.8 μM | 27077228 | |
| Raji cells | Antiproliferative activity assay | 48 h | IC50 = 19.3 μM | 27912175 | |
| Raji cells | Antiproliferative activity assay | 48 h | IC50 = 19.3 μM | 28432946 | |
| Raji cells | Antiproliferative activity assay | 72 h | IC50 = 19.5 μM | 30006143 | |
| Raji cells | Antiproliferative activity assay | 48 h | IC50 = 19.5 μM | 27956037 | |
| NAMALWA cells | Antiproliferative activity assay | 72 h | IC50 = 19.6 μM | 30006143 | |
| A2780 cells | Cytotoxicity assay | 3 days | EC50 = 20.1 μM | 27077228 | |
| Raji cells | Antiproliferative activity assay | 72 h | IC50 = 20.88 μM | 29146136 | |
| A549 cells | Antiproliferative activity assay | 72 h | IC50 = 21.79 μM | 28734581 | |
| SW480 cells | Cytotoxicity assay | 3 days | IC50 = 25.6 μM | 27077228 | |
| Ramos cells | Cytotoxicity assay | 24 h | IC50 = 28.7 μM | 28274675 | |
| sf9 cells | Function assay | IC50 = 0.0003 μM | 27994736 | ||
| MV411 cells | Growth inhibition assay | GI50 = 0.25 μM | 28315597 | ||
| M07e cells | Growth inhibition assay | GI50 = 0.59 μM | 28315597 | ||
| SU-DHL-2 cells | Growth inhibition assay | GI50 = 0.64 μM | 28315597 | ||
| Pfeiffer cells | Growth inhibition assay | GI50 = 1.6 μM | 28315597 | ||
| U937 cells | Growth inhibition assay | GI50 = 2.9 μM | 28315597 | ||
| NB4 cells | Growth inhibition assay | GI50 = 3 μM | 28315597 | ||
| Ramos cells | Growth inhibition assay | GI50 = 3.4 μM | 28315597 | ||
| SKM1 cells | Growth inhibition assay | GI50 = 3.6 μM | 28315597 | ||
| U2932 cells | Growth inhibition assay | GI50 = 4.4 μM | 28315597 | ||
| OCI-AML3 cells | Growth inhibition assay | GI50 = 9.2 μM | 28315597 | ||
| 他の多くの細胞株試験データをご覧になる場合はこちらをクリックして下さい | |||||
生物活性
| 製品説明 | イブルチニブ (Ibrutinib (PCI-32765)) は、無細胞アッセイで IC50 が 0.5 nM の強力で選択性の高い Brutons チロシンキナーゼ (Brutons tyrosine kinase, Btk) 阻害剤であり、Bmx, CSK, FGR, BRK, HCKに対しても阻害活性を持ちます。 EGFR, Yes, ErbB2, JAK3 に対しては前者より比較的弱い活性を持ちます。イブルチニブは、P13I を含む一連の PROTAC の合成における Btk リガンドとして適用できます。 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Targets |
|
| In Vitro | ||||
| In vitro |
Ibrutinib shows the potent and irreversible inhibitory effect and selectivity for Btk enzymatic activity. In BCR pathway-activated DOHH2 cell line, this compound inhibits autophosphorylation of Btk, phosphorylation of Btk's physiological substrate PLCγ, and phosphorylation of further downstream kinase, ERK with IC50 of 11 nM, 29 nM and 13 nM, respectively. It exhibits a significant dose-dependent and time-dependent induction of cytotoxicity in chronic lymphocytic leukemia (CLL) cells. In addition, this compound induces cell death depending on caspase pathway activation and antagonizes the ability of CLL cells to proliferate after TLR signaling. A recent study shows that this chemical inhibits BCR-activated primary B cell proliferation with IC50 of 8 nM and results in inhibition of TNFα, IL-1β and IL-6 production in primary monocytes with IC50 of 2.6 nM, 0.5 nM and 3.9 nM, respectively. |
|||
|---|---|---|---|---|
| Kinase Assay | Kinase Assays | |||
| In vitro kinase IC50 values are measured using 33P filtration binding assay after 1 hour incubation of kinase, 33P-ATP, Ibrutinib, and substrate [0.2 mg/mL poly(EY)(4:1]. Assays are performed at Reaction Biology. | ||||
| 細胞実験 | 細胞株 | Chronic lymphocytic leukemia (CLL) cells | ||
| 濃度 | 0.01-100 μM | |||
| 反応時間 | 48 hours | |||
| 実験の流れ | MTT (3'[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide) assays are performed to determine cytotoxicity. Briefly, cells (CLL B cells or healthy volunteer T cells or NK cells) are incubated for 48 hours with different concentrations of Ibrutinib, or vehicle control. MTT reagent is then added, and plates are incubated for an additional 20 hours before washing with protamine sulfate in phosphate-buffered saline. Dimethyl sulfoxide is added, and absorbance is measured by spectrophotometry at 540 nm in a Labsystems plate reader. Cell viability is also measured at various time points with the use of annexin/PI flow cytometry. Data are analyzed with Expo-ADC32 software package. Results are expressed as the percentage of total positive cells over untreated control. Experiments examining caspase-dependent apoptosis includes the addition of 100μM Z-VAD. |
|||
| 実験結果図 | Methods | Biomarkers | 結果図 | PMID |
| Western blot | pEGFR(Tyr1068) / EGFR pBTK / pPLCγ2 / pAKT / pERK / pJNK |
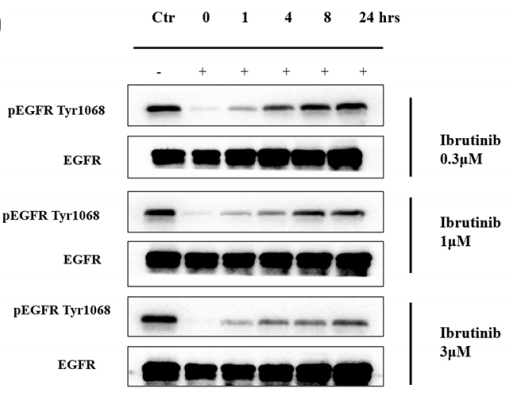
|
28061447 | |
| Immunofluorescence | CD11b COX-2 |
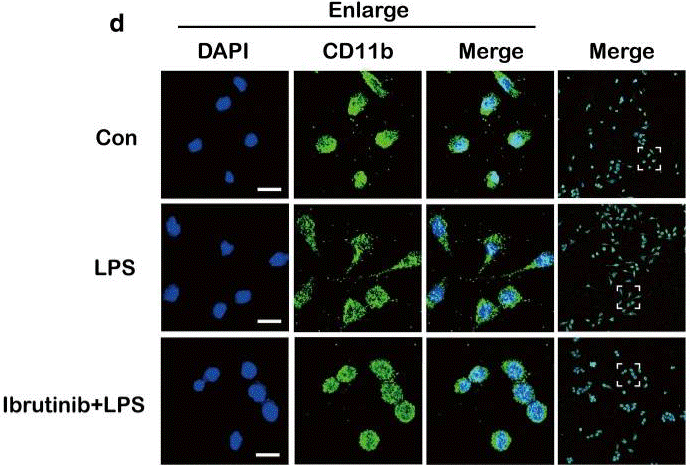
|
30231870 | |
| ELISA | hTNFα IL-10 |
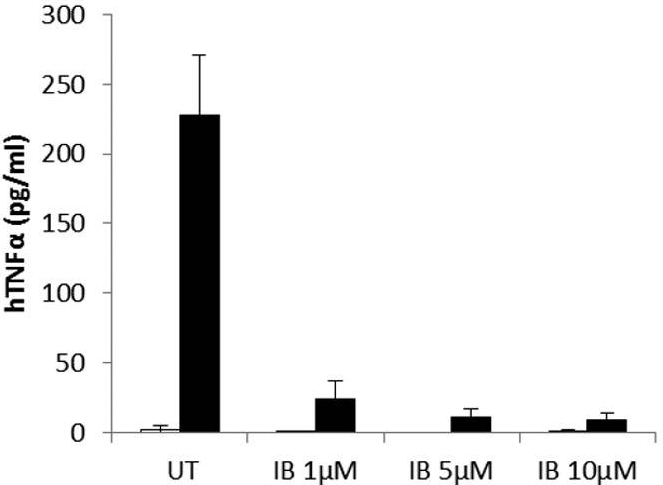
|
26627823 | |
| In Vivo | ||
| In Vivo |
In a collagen-induced arthritis model, Ibrutinib significantly reduces clinical arthritis scores reflecting paw swelling and joint inflammation by inhibiting B-cell activation. In a MRL-Fas(lpr) lupus model, this compound reduces renal disease and autoantibody production. In an adoptive transfer TCL1 mouse model of CLL, this compound (25 mg/kg/day) causes a transient early lymphocytosis, and delays CLL disease progression. |
|
|---|---|---|
| 動物実験 | 動物モデル | MRL-Fas(lpr) lupus model and collagen-induced arthritis model. |
| 投与量 | ≤50 mg/kg | |
| 投与経路 | Administered via p.o. | |
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06084923 | Not yet recruiting | Chronic Lymphocytic Leukemia |
Gruppo Italiano Malattie EMatologiche dell''Adulto |
May 2024 | -- |
| NCT06224452 | Not yet recruiting | Hematological Malignancy|Atrial Fibrillation |
University Hospital Caen |
March 1 2024 | -- |
| NCT05694312 | Recruiting | Autoimmune Hemolytic Anemia|Chronic Lymphocytic Leukemia|Small Lymphocytic Lymphoma|Monoclonal B-Cell Lymphocytosis CLL-Type |
Gruppo Italiano Malattie EMatologiche dell''Adulto |
November 24 2023 | Phase 2 |
|
化学情報
| 分子量 | 440.5 | 化学式 | C25H24N6O2 |
| CAS No. | 936563-96-1 | SDF | Download Ibrutinib (PCI-32765) SDFをダウンロードする |
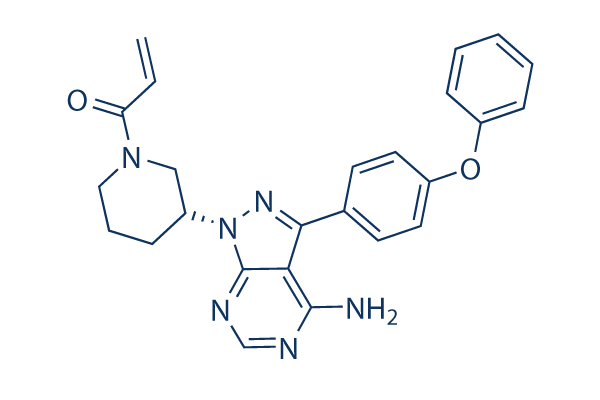
| Smiles | C=CC(=O)N1CCCC(C1)N2C3=NC=NC(=C3C(=N2)C4=CC=C(C=C4)OC5=CC=CC=C5)N | ||
| 保管 | |||
|
In vitro |
DMSO : 88 mg/mL ( (199.77 mM); 吸湿したDMSOは溶解度を減少させます。新しいDMSOをご使用ください。) Ethanol : 8 mg/mL Water : Insoluble |
モル濃度計算器 |
|
in vivo Add solvents to the product individually and in order. |
投与溶液組成計算機 | |||||
実験計算
投与溶液組成計算機(クリア溶液)
ステップ1:実験データを入力してください。(実験操作によるロスを考慮し、動物数を1匹分多くして計算・調製することを推奨します)
mg/kg
g
μL
匹
ステップ2:投与溶媒の組成を入力してください。(ロット毎に適した溶解組成が異なる場合があります。詳細については弊社までお問い合わせください)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
計算結果:
投与溶媒濃度: mg/ml;
DMSOストック溶液調製方法: mg 試薬を μL DMSOに溶解する(濃度 mg/mL, 注:濃度が当該ロットのDMSO溶解度を超える場合はご連絡ください。 )
投与溶媒調製方法:Take μL DMSOストック溶液に μL PEG300,を加え、完全溶解後μL Tween 80,を加えて完全溶解させた後 μL ddH2O,を加え完全に溶解させます。
投与溶媒調製方法:μL DMSOストック溶液に μL Corn oil,を加え、完全溶解。
注意:1.ストック溶液に沈殿、混濁などがないことをご確認ください;
2.順番通りに溶剤を加えてください。次のステップに進む前に溶液に沈殿、混濁などがないことを確認してから加えてください。ボルテックス、ソニケーション、水浴加熱など物理的な方法で溶解を早めることは可能です。
技術サポート
ストックの作り方、阻害剤の保管方法、細胞実験や動物実験の際に注意すべき点など、製品を取扱う時に問い合わせが多かった質問に対しては取扱説明書でお答えしています。
他に質問がある場合は、お気軽にお問い合わせください。
* 必須
よくある質問(FAQ)
質問1:
How to reconstitute it for in vivo studies?
回答
For in vivo study, we suggest to use 5% DMSO+30% PEG 300+5% Tween 80+ddH2O up to 10mg/ml for it.
