- 阻害剤
- 研究分野別
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- 化合物ライブラリー
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-II
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at info@selleck.co.jp to customize your library.
You could select:
- 抗体
- 新製品
- お問い合わせ
Imatinib (STI571) Mesylate
別名:CGP-57148B,STI571
Imatinib Mesylate is an orally bioavailability mesylate salt of Imatinib, which is a multi-target inhibitor of v-Abl, c-Kit and PDGFR with IC50 of 0.6 μM, 0.1 μM and 0.1 μM in cell-free or cell-based assays, respectively. Imatinib Mesylate (STI571) induces autophagy.
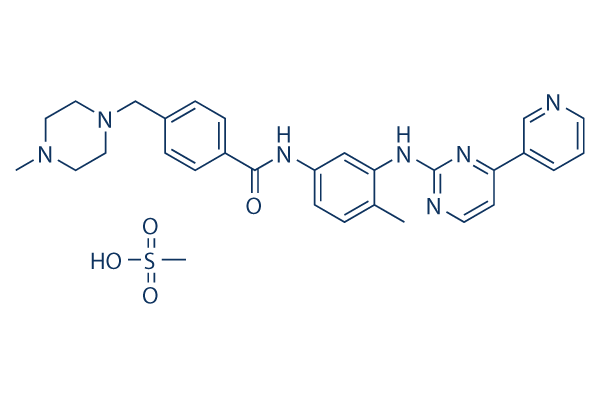
CAS No. 220127-57-1
文献中Selleckの製品使用例(252)
製品安全説明書
現在のバッチを見る:
純度:
99.99%
99.99
Imatinib (STI571) Mesylate関連製品
シグナル伝達経路
Bcr-Abl阻害剤の選択性比較
Cell Data
| Cell Lines | Assay Type | Concentration | Incubation Time | 活性情報 | PMID |
|---|---|---|---|---|---|
| MCF-7 | Growth Inhibition Assay | 10 μM | 48 h | blocks cell proliferation increase induced by BJ3Z | 25274034 |
| PC3 | Apoptosis Assay | 20 μM | 48/72 h | increases cell survival | 25786656 |
| PC3 | Cell Viability Assay | 20 μM | 6-72 h | increases cell viability | 25786656 |
| DU145 | Apoptosis Assay | 20 μM | 48/72 h | induces cell death by apoptosis | 25786656 |
| DU145 | Cell Viability Assay | 20 μM | 6-72 h | decreases cell viability | 25786656 |
| BA/F3 | Antiproliferative assay | 5 to 10 uM | 48 hrs | Antiproliferative activity against mouse BA/F3 cells expressing Tel-SH2-KD assessed as cell viability at 5 to 10 uM after 48 hrs by MTT assay, IC50 = 0.055 μM. | 16415863 |
| BA/F3 | Antiproliferative assay | 5 to 10 uM | 48 hrs | Antiproliferative activity against mouse BA/F3 cells expressing Tel-SH3-SH2-KD assessed as cell viability at 5 to 10 uM after 48 hrs by MTT assay, IC50 = 0.088 μM. | 16415863 |
| BA/F3 | Antiproliferative assay | 5 to 10 uM | 48 hrs | Antiproliferative activity against mouse BA/F3 cells expressing NPM-abl assessed as cell viability at 5 to 10 uM after 48 hrs by MTT assay, IC50 = 0.393 μM. | 16415863 |
| Ba/F3 | Function assay | 1 uM | 24 hrs | Inhibition of 14-3-3sigma in human Ba/F3 cells expressing wild type Bcr-Abl construct assessed as release of full-length c-Abl from cytoplasmic complex with 14-3-3sigma at 1 uM after 24 hrs by immunoprecipitation/immunoblot technique | 21962576 |
| Ba/F3 | Function assay | 1 uM | 24 hrs | Inhibition of 14-3-3sigma in human Ba/F3 cells expressing wild type Bcr-Abl construct assessed as nuclear import of full-length c-Abl at 1 uM after 24 hrs by immunoprecipitation/immunoblot technique | 21962576 |
| K562 | Function assay | 100 uM | 8 hrs | Inhibition of BCR-ABL signaling pathway in human K562 cells assessed as suppression of STAT5 phosphorylation at 100 uM after 8 hrs in presence of MeBS by Western blot analysis | 27666635 |
| K562 | Function assay | 100 uM | 8 hrs | Inhibition of BCR-ABL in signaling pathway human K562 cells assessed as suppression of CrkL phosphorylation at 100 uM after 8 hrs in presence of MeBS by Western blot analysis | 27666635 |
| K562 | Function assay | 100 uM | 8 hrs | Inhibition of BCR-ABL phosphorylation in human K562 cells at 100 uM after 8 hrs in presence of MeBS by Western blot analysis | 27666635 |
| K562 | Function assay | 10 uM | 1 hr | Inhibition of tyrosine phosphorylation of protein with molecular weight between 40 and 60 kDa in human K562 cells at 10 uM after 1 hr by immunoblot analysis | 24681986 |
| K562 | Function assay | 10 uM | 1 hr | Inhibition of Bcr/Abl kinase tyrosine phosphorylation in human K562 cells at 10 uM after 1 hr by immunoblot analysis | 24681986 |
| K562 | Function assay | 10 uM | 1 hr | Inhibition of Abl kinase tyrosine phosphorylation in human K562 cells at 10 uM after 1 hr by immunoblot analysis | 24681986 |
| NCI-H1703 | Function assay | 1 uM | 2 hrs | Inhibition of PDGF-induced PDGFRalpha autophosphorylation at Y754 residue in human NCI-H1703 cells at 1 uM after 2 hrs in presence of PDGF-AA by Western blot analysis | 29544149 |
| NCI-H1703 | Function assay | 1 uM | 2 hrs | Inhibition of PDGF-induced PDGFRalpha autophosphorylation at Y1018 residue in human NCI-H1703 cells at 1 uM after 2 hrs in presence of PDGF-AA by Western blot analysis | 29544149 |
| NCI-H1703 | Function assay | 1 uM | 2 hrs | Inhibition of PDGF-induced PDGFRalpha autophosphorylation at Y849 residue in human NCI-H1703 cells at 1 uM after 2 hrs in presence of PDGF-AA by Western blot analysis | 29544149 |
| EOL-1 | Function assay | 1 uM | 2 hrs | Inhibition of PDGFRalpha in human EOL-1 cells assessed as decrease in STAT5 phosphorylation at Y694 residue at 1 uM after 2 hrs in presence of PDGF-AA by Western blot analysis | 29544149 |
| EOL-1 | Function assay | 1 uM | 2 hrs | Inhibition of PDGFRalpha in human EOL-1 cells assessed as decrease in ERK phosphorylation at T202/Y204 residues at 1 uM after 2 hrs in presence of PDGF-AA by Western blot analysis | 29544149 |
| BA/F3 | Antitumor assay | 50 mg/kg | 14 days | Antitumor activity against mouse BA/F3 cells expressing wild type BCR-ABL allografted in SCID mouse assessed as inhibition of tumor growth at 50 mg/kg, po qd for 14 days | 23301703 |
| K562 | Function assay | 40 uM | 6 hrs | Inhibition of BCR-ABL-mediated ERK1 phosphorylation in human K562 cells at 40 uM after 6 hrs by phospho-flow cytometry | 22148584 |
| K562 | Function assay | 40 uM | 6 hrs | Inhibition of BCR-ABL-mediated ERK2 phosphorylation in human K562 cells at 40 uM after 6 hrs by phospho-flow cytometry | 22148584 |
| GISTT1 | Function assay | 1 uM | 2 hrs | Inhibition of c-KIT autophosphorylation at Y719 in human GISTT1 cells at 1 uM measured after 2 hrs by immunoblotting | 27966954 |
| GISTT1 | Function assay | 1 uM | 2 hrs | Inhibition of c-KIT autophosphorylation at Y823 in human GISTT1 cells at 1 uM measured after 2 hrs by immunoblotting | 27966954 |
| GISTT1 | Function assay | 1 uM | 2 hrs | Inhibition of c-KIT autophosphorylation at Y703 in human GISTT1 cells at 1 uM measured after 2 hrs by immunoblotting | 27966954 |
| GIST882 | Function assay | 1 uM | 2 hrs | Inhibition of c-KIT autophosphorylation at Y823 in human GIST882 cells at 1 uM measured after 2 hrs by immunoblotting | 27966954 |
| GIST882 | Function assay | 1 uM | 2 hrs | Inhibition of c-KIT autophosphorylation at Y703 in human GIST882 cells at 1 uM measured after 2 hrs by immunoblotting | 27966954 |
| GIST882 | Function assay | 1 uM | 2 hrs | Inhibition of c-KIT autophosphorylation at Y719 in human GIST882 cells at 1 uM measured after 2 hrs by immunoblotting | 27966954 |
| GISTT1 | Function assay | 1 uM | 2 hrs | Inhibition of c-KIT mediated signaling pathways assessed as reduction in AKT phosphorylation at S473 in human GISTT1 cells at 1 uM measured after 2 hrs by immunoblotting | 27966954 |
| GISTT1 | Function assay | 1 uM | 2 hrs | Inhibition of c-KIT mediated signaling pathways assessed as reduction in Stat3/5 phosphorylation at Y705/Y694 in human GISTT1 cells at 1 uM measured after 2 hrs by immunoblotting | 27966954 |
| GISTT1 | Function assay | 1 uM | 2 hrs | Inhibition of c-KIT mediated signaling pathways assessed as reduction in ERK1/2 phosphorylation at T202/Y204 in human GISTT1 cells at 1 uM measured after 2 hrs by immunoblotting | 27966954 |
| GISTT1 | Function assay | 1 uM | 2 hrs | Inhibition of c-KIT mediated signaling pathways assessed as reduction in S6 phosphorylation at S235/236 in human GISTT1 cells at 1 uM measured after 2 hrs by immunoblotting | 27966954 |
| GIST882 | Function assay | 1 uM | 2 hrs | Inhibition of c-KIT mediated signaling pathways assessed as reduction in S6 phosphorylation at S235/236 in human GIST882 cells at 1 uM measured after 2 hrs by immunoblotting | 27966954 |
| GIST882 | Cell cycle assay | 1 uM | 48 hrs | Cell cycle arrest in human GIST882 cells assessed as accumulation at G0/G1 phase at 1 uM after 48 hrs by propidium iodide/RNase staining based flow cytometry | 27966954 |
| K562 | Cell cycle assay | 1 uM | 24 hrs | Cell cycle arrest in human K562 cells assessed as accumulation at G0/G1 phase at 1 uM after 24 hrs by propidium iodide/RNase staining based flow cytometry | 27966954 |
| GISTT1 | Function assay | 1 uM | 2 hrs | Inhibition of c-KIT mediated signaling pathways assessed as reduction in S6K phosphorylation at T389 in human GISTT1 cells at 1 uM measured after 2 hrs by immunoblotting | 27966954 |
| GIST882 | Function assay | 1 uM | 2 hrs | Inhibition of c-KIT mediated signaling pathways assessed as reduction in Stat3/5 phosphorylation at Y705/Y694 in human GIST882 cells at 1 uM measured after 2 hrs by immunoblotting | 27966954 |
| GIST882 | Function assay | 1 uM | 2 hrs | Inhibition of c-KIT mediated signaling pathways assessed as reduction in ERK1/2 phosphorylation at T202/Y204 in human GIST882 cells at 1 uM measured after 2 hrs by immunoblotting | 27966954 |
| GIST882 | Function assay | 1 uM | 2 hrs | Inhibition of c-KIT mediated signaling pathways assessed as reduction in S6K phosphorylation at T389 in human GIST882 cells at 1 uM measured after 2 hrs by immunoblotting | 27966954 |
| KU812 | Cell cycle assay | 1 uM | 12 hrs | Cell cycle arrest in human KU812 cells assessed as accumulation at G0/G1 phase at 1 uM after 12 hrs by propidium iodide/RNase staining based flow cytometry | 27966954 |
| MEG01 | Cell cycle assay | 1 uM | 24 hrs | Cell cycle arrest in human MEG01 cells assessed as accumulation at G0/G1 phase at 1 uM after 24 hrs by propidium iodide/RNase staining based flow cytometry | 27966954 |
| GISTT1 | Cell cycle assay | 1 uM | 24 hrs | Cell cycle arrest in human GISTT1 cells assessed as accumulation at G0/G1 phase at 1 uM after 24 hrs by propidium iodide/RNase staining based flow cytometry | 27966954 |
| GIST882 | Function assay | 1 uM | 2 hrs | Inhibition of c-KIT mediated signaling pathways assessed as reduction in AKT phosphorylation at S473 in human GIST882 cells at 1 uM measured after 2 hrs by immunoblotting | 27966954 |
| Neuro2a | Function assay | 1 uM | Inhibition of DR24 in Dhcr7-deficient mouse Neuro2a cells assessed as decrease in 7-DHC levels at 1 uM by LC-MS/GC-MS analysis | 26789657 | |
| K562 | Function assay | 1 uM | Inhibition of BCR-ABL in imatinib-sensitive human K562 cells assessed as decrease in CrKL phosphorylation at 1 uM by immuno blot analysis | 30261468 | |
| K562 | Apoptosis assay | 1 uM | Induction of apoptosis in human K562 cells assessed as cleavage of PARP at 1 uM by Western blot analysis | 27966954 | |
| K562 | Apoptosis assay | 1 uM | Induction of apoptosis in human K562 cells assessed as increase in cleaved caspase-3 level at 1 uM by Western blot analysis | 27966954 | |
| KU812 | Apoptosis assay | 1 uM | Induction of apoptosis in human KU812 cells assessed as cleavage of PARP at 1 uM by Western blot analysis | 27966954 | |
| KU812 | Apoptosis assay | 1 uM | Induction of apoptosis in human KU812 cells assessed as increase in cleaved caspase-3 level at 1 uM by Western blot analysis | 27966954 | |
| MEG01 | Apoptosis assay | 1 uM | Induction of apoptosis in human MEG01 cells assessed as cleavage of PARP at 1 uM by Western blot analysis | 27966954 | |
| GISTT1 | Apoptosis assay | 1 uM | Induction of apoptosis in human GISTT1 cells assessed as cleavage of PARP at 1 uM by Western blot analysis | 27966954 | |
| GIST882 | Apoptosis assay | 1 uM | Induction of apoptosis in human GIST882 cells assessed as cleavage of PARP at 1 uM by Western blot analysis | 27966954 | |
| GIST882 | Apoptosis assay | 1 uM | Induction of apoptosis in human GIST882 cells assessed as increase in cleaved caspase-3 level at 1 uM by Western blot analysis | 27966954 | |
| MEG01 | Apoptosis assay | 1 uM | Induction of apoptosis in human MEG01 cells assessed as increase in cleaved caspase-3 level at 1 uM by Western blot analysis | 27966954 | |
| GISTT1 | Apoptosis assay | 1 uM | Induction of apoptosis in human GISTT1 cells assessed as increase in cleaved caspase-3 level at 1 uM by Western blot analysis | 27966954 | |
| MDA-MB-23 | Cytotoxicity Assay | 24 h | IC50=1.8 μM | 22000207 | |
| MCF-7 | Cytotoxicity Assay | 24 h | IC50=0.83 μM | 22000207 | |
| K562 | Cytotoxicity Assay | 24 h | IC50=0.21 μM | 22000207 | |
| GIST882 | Growth Inhibition Assay | 96 h | IC50=1.7 μM | 24900212 | |
| EOL-1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human EOL-1 cells after 72 hrs by alamar-blue cell viability assay, IC50 = 0.0002 μM. | 19301902 | |
| EOL-1 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human EOL-1 cells after 72 hrs by CellTiter-Glo or CCK-8 assay, GI50 = 0.001 μM. | 29544149 | |
| GISTT1 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human GISTT1 cells assessed as cell growth inhibition after 72 hrs by cell titer-glo assay, GI50 = 0.003 μM. | 28541695 | |
| GISTT1 | Antiproliferative assay | 72 hrs | Antiproliferative activity against cKIT dependent human GISTT1 cells assessed as reduction in cell viability after 72 hrs by celltiter-glo/CCK8 assay, GI50 = 0.008 μM. | 27077705 | |
| GISTT1 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human GISTT1 cells measured after 72 hrs by CellTiter-Glo or CCK8 assay, GI50 = 0.008 μM. | 27966954 | |
| GISTT1 | Function assay | 72 hrs | Inhibition of KIT in human GISTT1 cells assessed as growth inhibition after 72 hrs by SRB assay, GI50 = 0.01 μM. | 23773153 | |
| BAF3 | Function assay | 72 hrs | Inhibition of TEL-fused PDGFRalpha (unknown origin) expressed in mouse BAF3 cells assessed as cell growth inhibition after 72 hrs by cell titer-glo assay, GI50 = 0.0107 μM. | 28541695 | |
| GIST882 | Antiproliferative assay | 72 hrs | Antiproliferative activity against cKIT dependent human GIST882 cells assessed as reduction in cell viability after 72 hrs by celltiter-glo/CCK8 assay, GI50 = 0.014 μM. | 27077705 | |
| GIST882 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human GIST882 cells measured after 72 hrs by CellTiter-Glo or CCK8 assay, GI50 = 0.014 μM. | 27966954 | |
| BA/F3 | Function assay | 72 hrs | Inhibition of Kit exon 11 deletion (557 to 558 residues) mutant (unknown origin) transfected in mouse BA/F3 cells assessed as inhibition of cell growth incubated for 72 hrs by MTS assay, GI50 = 0.017 μM. | 30204441 | |
| BA/F3 | Function assay | 72 hrs | Inhibition of TEL-fused PDGFRbeta (unknown origin) expressed in mouse BA/F3 cells assessed as growth inhibition after 72 hrs by CellTiter-Glo or CCK-8 assay, GI50 = 0.019 μM. | 29544149 | |
| BAF3 | Function assay | 72 hrs | Inhibition of Tel-fused PDGFR-beta (unknown origin) expressed in BAF3 cells assessed as growth inhibition measured after 72 hrs by CellTiter-Glo or CCK8 assay, GI50 = 0.019 μM. | 27966954 | |
| GIST882 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human GIST882 cells assessed as cell growth inhibition after 72 hrs by cell titer-glo assay, GI50 = 0.02 μM. | 28541695 | |
| BAF3 | Function assay | 72 hrs | Inhibition of TEL-fused cKIT C674S mutant (unknown origin) expressed in mouse BAF3 cells assessed as cell growth inhibition after 72 hrs by cell titer-glo assay, GI50 = 0.0249 μM. | 28541695 | |
| BA/F3 | Antiproliferative assay | 48 hrs | Antiproliferative activity against mouse BA/F3 Bcr-abl negative cells expressing Tel-PDGFRbeta kinase assessed as proliferation after 48 hrs by MTT assay, IC50 = 0.027 μM. | 16415863 | |
| HL60 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HL60 cells assessed as inhibition of cell survival after 72 hrs by MTT assay, IC50 = 0.03 μM. | 26850004 | |
| BAF3 | Function assay | 72 hrs | Inhibition of human wild type BCR-ABL expressed in mouse BAF3 cells assessed as inhibition of cell proliferation after 72 hrs by MTT assay, EC50 = 0.034 μM. | 27010810 | |
| BA/F3 | Function assay | 72 hrs | Inhibition of TEL-fused PDGFRalpha (unknown origin) expressed in mouse BA/F3 cells assessed as growth inhibition after 72 hrs by CellTiter-Glo or CCK-8 assay, GI50 = 0.034 μM. | 29544149 | |
| BAF3 | Function assay | 72 hrs | Inhibition of Tel-fused PDGFR-alpha (unknown origin) expressed in BAF3 cells assessed as growth inhibition measured after 72 hrs by CellTiter-Glo or CCK8 assay, GI50 = 0.034 μM. | 27966954 | |
| BAF3 | Function assay | 72 hrs | Inhibition of TEL-fused cKIT V559D mutant (unknown origin) expressed in mouse BAF3 cells assessed as cell growth inhibition after 72 hrs by cell titer-glo assay, GI50 = 0.036 μM. | 28541695 | |
| BAF3 | Function assay | 72 hrs | Inhibition of Tel-fused cKIT V559D mutant (unknown origin) transfected in mouse BAF3 cells assessed as decrease in cell proliferation after 72 hrs by celltiter-glo/CCK8 assay, GI50 = 0.039 μM. | 27077705 | |
| BAF3 | Function assay | 72 hrs | Inhibition of Tel-fused c-KIT V559D mutant (unknown origin) expressed in BAF3 cells assessed as growth inhibition measured after 72 hrs by CellTiter-Glo or CCK8 assay, GI50 = 0.039 μM. | 27966954 | |
| MDCK2 | Function assay | 5 mins | Inhibition of human MATE1-mediated [14]-metformin uptake expressed in polarized MDCK2 cells after 5 mins by liquid scintillation counting analysis, IC50 = 0.04 μM. | 23241029 | |
| GISTT1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human GISTT1 cells assessed as cell growth inhibition after 72 hrs by CellTiterGlo assay, GI50 = 0.04 μM. | 28991465 | |
| K562 | Function assay | 30 mins | Inhibition of kinobead binding to NQO2 in human K562 cells incubated for 30 mins by iTRAQ reagent-based mass spectrometric method, IC50 = 0.043 μM. | 28280261 | |
| MEG01 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MEG01 cells assessed as cell growth inhibition after 72 hrs by cell titer-glo assay, GI50 = 0.045 μM. | 28541695 | |
| BAF3 | Function assay | 72 hrs | Inhibition of TEL-fused cKIT L576P mutant (unknown origin) expressed in mouse BAF3 cells assessed as cell growth inhibition after 72 hrs by cell titer-glo assay, GI50 = 0.0451 μM. | 28541695 | |
| BA/F3 | Function assay | 72 hrs | Inhibition of TEL-fused PDGFRalpha (unknown origin) transfected in mouse BA/F3 cells assessed as inhibition of cell growth incubated for 72 hrs by MTS assay, GI50 = 0.046 μM. | 30204441 | |
| K562 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human K562 cells after 72 hrs by alamar-blue cell viability assay, IC50 = 0.049 μM. | 19301902 | |
| HEK293 | Function assay | 1.5 mins | Inhibition of human MATE1-mediated [14]-metformin uptake expressed in HEK293 cells after 1.5 mins by scintillation counting analysis, IC50 = 0.05 μM. | 23241029 | |
| HT-29 | Antiproliferative assay | 96 hrs | Antiproliferative activity against human HT-29 cells after 96 hrs by SRB assay, IC50 = 0.06 μM. | 19469547 | |
| HT-29 | Antiproliferative assay | 96 hrs | Antiproliferative activity against human HT-29 cells after 96 hrs by SRB assay, IC50 = 0.06 μM. | 29724653 | |
| JURL-MK1 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human JURL-MK1 cells after 48 hrs by Cell-Titer-Glo luminescent cell viability assay, GI50 = 0.06 μM. | 27011159 | |
| BA/F3 | Function assay | 72 hrs | Inhibition of Kit V560D mutant (unknown origin) transfected in mouse BA/F3 cells assessed as inhibition of cell growth incubated for 72 hrs by MTS assay, GI50 = 0.07 μM. | 30204441 | |
| MEG01 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human BCR-ABL dependent MEG01 cells assessed as reduction in cell viability after 72 hrs by celltiter-glo/CCK8 assay, GI50 = 0.074 μM. | 27077705 | |
| MEG01 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MEG01 cells measured after 72 hrs by CellTiter-Glo or CCK8 assay, GI50 = 0.074 μM. | 27966954 | |
| Ba/F3 | Function assay | 48 hrs | Inhibition of BCR-ABL1 G250H mutant (unknown origin) expressed in mouse Ba/F3 cells assessed as inhibition of BCR-ABL1-mediated cell proliferation after 48 hrs by BriteLight luciferase assay, GI50 = 0.0771 μM. | 30137981 | |
| BA/F3 | Growth inhibition assay | 48 hrs | Growth inhibition of mouse BA/F3 cells expressing wild-type Bcr-Abl after 48 hrs by MTT assay, IC50 = 0.089 μM. | 26562217 | |
| K562 | Function assay | 30 mins | Inhibition of kinobead binding to DDR1 in human K562 cells incubated for 30 mins by iTRAQ reagent-based mass spectrometric method, IC50 = 0.09 μM. | 28280261 | |
| Ba/F3 | Function assay | 48 hrs | Inhibition of wild type BCR-ABL1 (unknown origin) expressed in mouse Ba/F3 cells assessed as inhibition of cell proliferation after 48 hrs by BriteLight luciferase assay, GI50 = 0.0905 μM. | 30137981 | |
| BA/F3 | Cytotoxicity assay | 48 hrs | Cytotoxicity against mouse BA/F3 cells transfected with wild type Bcr-Abl after 48 hrs by XTT assay, IC50 = 0.092 μM. | 23600806 | |
| insect | Function assay | 30 mins | Inhibition of human recombinant wild type ABL1 expressed in insect cells after 30 mins by FRET assay, IC50 = 0.0982 μM. | 23301703 | |
| K562 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human K562 cells after 72 hrs by alamar-blue cell viability assay, IC50 = 0.1 μM. | 19301902 | |
| primary leukemia cells | Cytotoxicity assay | 72 hrs | Cytotoxicity against human primary leukemia cells isolated from acute myeloid leukemia patient expressing wild type FLT3 assessed as cell viability after 72 hrs by luciferase assay, IC50 = 0.1 μM. | 22221201 | |
| BV173 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human BV173 cells after 48 hrs by Cell-Titer-Glo luminescent cell viability assay, GI50 = 0.1 μM. | 27011159 | |
| BAF3 | Function assay | 72 hrs | Inhibition of Tel-fused cKIT L567P mutant (unknown origin) transfected in mouse BAF3 cells assessed as decrease in cell proliferation after 72 hrs by celltiter-glo/CCK8 assay, GI50 = 0.1 μM. | 27077705 | |
| K562 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human K562 cells after 24 hrs by MTT assay, IC50 = 0.1 μM. | 22632935 | |
| BA/F3 | Function assay | 72 hrs | Inhibition of TEL-fused PDGFRbeta (unknown origin) transfected in mouse BA/F3 cells assessed as inhibition of cell growth incubated for 72 hrs by MTS assay, GI50 = 0.102 μM. | 30204441 | |
| BAF3 | Function assay | 72 hrs | Inhibition of Tel-fused c-KIT L576P mutant (unknown origin) expressed in BAF3 cells assessed as growth inhibition measured after 72 hrs by CellTiter-Glo or CCK8 assay, GI50 = 0.102 μM. | 27966954 | |
| KU812 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human KU812 cells assessed as cell growth inhibition after 72 hrs by cell titer-glo assay, GI50 = 0.11 μM. | 28541695 | |
| BA/F3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against mouse BA/F3 cells expressing Bcr-Abl H396P mutant after 72 hrs by CCK-8 assay, IC50 = 0.11 μM. | 23088644 | |
| BAF3 | Function assay | 72 hrs | Inhibition of Tel-fused CSF1R (unknown origin) expressed in BAF3 cells assessed as growth inhibition measured after 72 hrs by CellTiter-Glo or CCK8 assay, GI50 = 0.11 μM. | 27966954 | |
| 32D | Antiproliferative assay | 48 hrs | Antiproliferative activity against mouse 32D cells transfected with p210 cells expressing Bcr-abl assessed as proliferation after 48 hrs by MTT assay, IC50 = 0.112 μM. | 16415863 | |
| insect cells | Function assay | 30 mins | Inhibition of human recombinant ABL1 M351T mutant expressed in insect cells after 30 mins by FRET assay, IC50 = 0.1143 μM. | 23301703 | |
| insect cells | Function assay | 30 mins | Inhibition of human recombinant ABL1 Q252H mutant expressed in insect cells after 30 mins by FRET assay, IC50 = 0.115 μM. | 23301703 | |
| K562 | Antiproliferative assay | 72 hrs | Antiproliferative activity against BCR-ABL dependent human K562 cells assessed as reduction in cell viability after 72 hrs by celltiter-glo/CCK8 assay, GI50 = 0.12 μM. | 27077705 | |
| BA/F3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against mouse BA/F3 cells expressing Bcr-Abl M244V mutant after 72 hrs by CCK-8 assay, IC50 = 0.129 μM. | 23088644 | |
| K562 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human K562 cells assessed as cell viability after 72 hrs by CellTiter-Glo or CCK-8 assay, GI50 = 0.14 μM. | 26789553 | |
| K562 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human K562 cells assessed as cell growth inhibition after 72 hrs by cell titer-glo assay, GI50 = 0.147 μM. | 28541695 | |
| BAF3 | Function assay | 72 hrs | Inhibition of TEL-fused cKIT A829P mutant (unknown origin) expressed in mouse BAF3 cells assessed as cell growth inhibition after 72 hrs by cell titer-glo assay, GI50 = 0.1482 μM. | 28541695 | |
| Ba/F3 | Function assay | 48 hrs | Inhibition of BCR-ABL1 E355G mutant (unknown origin) expressed in mouse Ba/F3 cells assessed as inhibition of BCR-ABL1-mediated cell proliferation after 48 hrs by BriteLight luciferase assay, GI50 = 0.149 μM. | 30137981 | |
| KU812 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human KU812 cells assessed as cell viability after 72 hrs by CellTiter-Glo or CCK-8 assay, GI50 = 0.16 μM. | 26789553 | |
| KU812 | Antiproliferative assay | 72 hrs | Antiproliferative activity against BCR-ABL dependent human KU812 cells assessed as reduction in cell viability after 72 hrs by celltiter-glo/CCK8 assay, GI50 = 0.16 μM. | 27077705 | |
| A10 | Function assay | 68 hrs | Inhibition of PDGFR-beta driven proliferation of rat A10 cells after 68 hrs in presence of rat recombinant PDGF-BB by cell titer-glo luminescence assay, IC50 = 0.162 μM. | 27502700 | |
| KU812 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human KU812 cells measured after 72 hrs by CellTiter-Glo or CCK8 assay, GI50 = 0.163 μM. | 27966954 | |
| insect cells | Function assay | 30 mins | Inhibition of human recombinant ABL1 H396P mutant expressed in insect cells after 30 mins by FRET assay, IC50 = 0.1739 μM. | 23301703 | |
| BA/F3 | Antiproliferative assay | 48 hrs | Antiproliferation activity against mouse BA/F3 cells expressing Bcr-abl assessed as cell viability after 48 hrs by MTT assay, IC50 = 0.19 μM. | 16415863 | |
| BA/F3 | Antiproliferative assay | 48 hrs | Antiproliferative activity against mouse BA/F3 cells transfected with p210 Bcr-abl assessed as proliferation after 48 hrs by MTT assay, IC50 = 0.19 μM. | 16415863 | |
| BA/F3 | Function assay | 72 hrs | Inhibition of Kit exon 9 AY502 to 503 insertion mutant (unknown origin) transfected in mouse BA/F3 cells assessed as inhibition of cell growth incubated for 72 hrs by MTS assay, GI50 = 0.192 μM. | 30204441 | |
| K562 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human K562 cells after 48 hrs by Cell-Titer-Glo luminescent cell viability assay, GI50 = 0.2 μM. | 27011159 | |
| Ba/F3 | Function assay | 48 hrs | Inhibition of BCR-ABL1 E459K mutant (unknown origin) expressed in mouse Ba/F3 cells assessed as inhibition of BCR-ABL1-mediated cell proliferation after 48 hrs by BriteLight luciferase assay, GI50 = 0.201 μM. | 30137981 | |
| BA/F3 | Function assay | 72 hrs | Inhibition of Kit exon 11 deletion (557 to 558 residues) and D820A mutant and D820A mutant (unknown origin) transfected in mouse BA/F3 cells assessed as inhibition of cell growth incubated for 72 hrs by MTS assay, GI50 = 0.216 μM. | 30204441 | |
| K562 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human K562 cells after 48 hrs by trypan blue dye exclusion assay, IC50 = 0.22 μM. | 26629859 | |
| BA/F3 | Function assay | 72 hrs | Inhibition of Kit exon 11 deletion (557 to 558 residues) and N822K mutant and Y823D mutant (unknown origin) transfected in mouse BA/F3 cells assessed as inhibition of cell growth incubated for 72 hrs by MTS assay, GI50 = 0.223 μM. | 30204441 | |
| SF9 | Function assay | 1 hr | Inhibition of C-terminal His-tagged human ABL1 expressed in baculovirus infected SF9 cells using Tyr 02 peptide as substrate measured after 1 hr by FRET based Z'Lyte assay, IC50 = 0.223 μM. | 27966954 | |
| NCI-H1703 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human NCI-H1703 cells after 72 hrs in presence of PDGF-AA by CellTiter-Glo or CCK-8 assay, GI50 = 0.23 μM. | 29544149 | |
| MEG01 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MEG01 cells assessed as cell viability after 72 hrs by CellTiter-Glo or CCK-8 assay, GI50 = 0.24 μM. | 26789553 | |
| BA/F3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against mouse BA/F3 cells expressing BCR-ABL M351T mutant assessed as growth inhibition after 72 hrs by CCK-8 assay, IC50 = 0.24 μM. | 23301703 | |
| Ba/F3 | Function assay | 48 hrs | Inhibition of BCR-ABL1 F359V mutant (unknown origin) expressed in mouse Ba/F3 cells assessed as inhibition of BCR-ABL1-mediated cell proliferation after 48 hrs by BriteLight luciferase assay, GI50 = 0.249 μM. | 30137981 | |
| K562 | Function assay | 30 mins | Inhibition of kinobead binding to ABL in human K562 cells incubated for 30 mins by iTRAQ reagent-based mass spectrometric method, IC50 = 0.25 μM. | 28280261 | |
| M07e | Antiproliferative assay | 48 hrs | Antiproliferative activity against human M07e Bcr-abl negative cells expressing stem cell factor assessed as proliferation after 48 hrs by MTT assay, IC50 = 0.257 μM. | 16415863 | |
| EM2 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human EM2 cells after 48 hrs by Cell-Titer-Glo luminescent cell viability assay, GI50 = 0.26 μM. | 27011159 | |
| K562 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human K562 cells measured after 72 hrs by CellTiter-Glo or CCK8 assay, GI50 = 0.267 μM. | 27966954 | |
| BAF3 | Function assay | 72 hrs | Inhibition of human BCR/ABL p210 fusion protein expressed in BAF3 cells assessed as growth inhibition measured after 72 hrs by CellTiter-Glo or CCK8 assay, GI50 = 0.27 μM. | 27966954 | |
| K562 | Function assay | 30 mins | Inhibition of kinobead binding to ARG in human K562 cells incubated for 30 mins by iTRAQ reagent-based mass spectrometric method, IC50 = 0.272 μM. | 28280261 | |
| KBM5 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human KBM5 cells harboring wild type Bcr-Abl after 72 hrs by MTT assay, IC50 = 0.28 μM. | 20149665 | |
| K562 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human K562 cells expressing wild type Bcr-Abl after 72 hrs by CCK-8 assay, IC50 = 0.28 μM. | 26195136 | |
| K562 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human K562 cells assessed as decrease in cell proliferation after 72 hrs by XTT assay, IC50 = 0.31 μM. | 27214512 | |
| BA/F3 | Function assay | 72 hrs | Inhibition of Kit exon 11 deletion (557 to 558 residues) and A829P mutant and Y823D mutant (unknown origin) transfected in mouse BA/F3 cells assessed as inhibition of cell growth incubated for 72 hrs by MTS assay, GI50 = 0.312 μM. | 30204441 | |
| BA/F3 | Antiproliferative assay | 48 hrs | Antiproliferation activity against mouse BA/F3 cells expressing Bcr-abl A337N mutant assessed as cell viability after 48 hrs by MTT assay, IC50 = 0.339 μM. | 16415863 | |
| HEK293 | Function assay | 1.5 mins | Inhibition of human MATE2K-mediated ASP+ uptake expressed in HEK293 cells up to 500 uM after 1.5 mins by fluorescence assay, IC50 = 0.35 μM. | 23241029 | |
| HEK293 | Function assay | 1.5 mins | Inhibition of human MATE1-mediated ASP+ uptake expressed in HEK293 cells after 1.5 mins by fluorescence assay, IC50 = 0.35 μM. | 23241029 | |
| insect cells | Function assay | 30 mins | Inhibition of human recombinant ABL1 G250E mutant expressed in insect cells after 30 mins by FRET assay, IC50 = 0.3599 μM. | 23301703 | |
| BA/F3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against mouse BA/F3 cells expressing Bcr-Abl E355G mutant after 72 hrs by CCK-8 assay, IC50 = 0.362 μM. | 23088644 | |
| BA/F3 | Function assay | 72 hrs | Inhibition of TEL-fused c-KIT (unknown origin) expressed in mouse BA/F3 cells assessed as growth inhibition after 72 hrs by CellTiter-Glo or CCK-8 assay, GI50 = 0.37 μM. | 29544149 | |
| K562 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human K562 cells assessed as inhibition of cell survival after 72 hrs by MTT assay, IC50 = 0.38 μM. | 26850004 | |
| BA/F3 | Function assay | 72 hrs | Inhibition of BCR/ABL p210 (unknown origin) expressed in mouse BA/F3 cells assessed as growth inhibition after 72 hrs by CellTiter-Glo or CCK-8 assay, GI50 = 0.38 μM. | 26789553 | |
| BAF3 | Function assay | 72 hrs | Inhibition of TEL-fused cKIT (unknown origin) expressed in mouse BAF3 cells assessed as cell growth inhibition after 72 hrs by cell titer-glo assay, GI50 = 0.3819 μM. | 28541695 | |
| K562 | Cytotoxicity assay | 48 to 72 hrs | Cytotoxicity against human BCR-ABL positive K562 cells after 48 to 72 hrs by MTT assay, IC50 = 0.3844 μM. | 22789429 | |
| K562 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human K562 cells after 72 hrs by MTS assay, GI50 = 0.39 μM. | 22932313 | |
| BA/F3 | Function assay | 72 hrs | Inhibition of Kit exon 11 deletion (557 to 558 residues) and V654A mutant (unknown origin) transfected in mouse BA/F3 cells assessed as inhibition of cell growth incubated for 72 hrs by MTS assay, GI50 = 0.392 μM. | 30204441 | |
| BAF3 | Function assay | 72 hrs | Inhibition of Tel-fused cKIT (unknown origin) transfected in mouse BAF3 cells assessed as decrease in cell proliferation after 72 hrs by celltiter-glo/CCK8 assay, GI50 = 0.4 μM. | 27077705 | |
| BA/F3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against mouse BA/F3 cells expressing Bcr-Abl M351T mutant after 72 hrs by CCK-8 assay, IC50 = 0.424 μM. | 23088644 | |
| KCL22 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human KCL22 cells after 48 hrs by Cell-Titer-Glo luminescent cell viability assay, GI50 = 0.43 μM. | 27011159 | |
| Ba/F3 | Function assay | 48 hrs | Inhibition of BCR-ABL1 Q252H mutant (unknown origin) expressed in mouse Ba/F3 cells assessed as inhibition of BCR-ABL1-mediated cell proliferation after 48 hrs by BriteLight luciferase assay, GI50 = 0.455 μM. | 30137981 | |
| K562 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human K562 cells assessed as growth inhibition after 48 hrs by MTT assay, IC50 = 0.47 μM. | 23735826 | |
| K562 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human K562 cells after 48 hrs by MTT assay, IC50 = 0.47 μM. | 25778766 | |
| insect cells | Function assay | 30 mins | Inhibition of human recombinant ABL1 E255K mutant expressed in insect cells after 30 mins by FRET assay, IC50 = 0.4858 μM. | 23301703 | |
| primary leukemia cells | Cytotoxicity assay | 72 hrs | Cytotoxicity against human primary leukemia cells isolated from acute myeloid leukemia patient expressing FLT3-D835Y mutation assessed as cell viability after 72 hrs by luciferase assay, IC50 = 0.5 μM. | 22221201 | |
| BA/F3 | Function assay | 72 hrs | Inhibition of TEL-LCK (unknown origin) expressed in mouse BA/F3 cells assessed as growth inhibition after 72 hrs by CellTiter-Glo or CCK-8 assay, GI50 = 0.5 μM. | 26789553 | |
| K562 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human K562 cells after 72 hrs by calcein-AM assay, IC50 = 0.5 μM. | 23981532 | |
| K562 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human K562 cells after 72 hrs using Calcein AM by fluorescence assay, IC50 = 0.5 μM. | 25757603 | |
| BA/F3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against mouse BA/F3 cells expressing wild type BCR-ABL assessed as growth inhibition after 72 hrs by CCK-8 assay, IC50 = 0.5 μM. | 23301703 | |
| K562 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human K562 cells after 48 hrs by MTT assay, IC50 = 0.5 μM. | 23932071 | |
| K562 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human K562 cells assessed as cell viability after 72 hrs by MTT assay, IC50 = 0.51 μM. | 26814890 | |
| K562 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human K562 cells after 48 hrs by MTT assay, IC50 = 0.53 μM. | 26707846 | |
| K562 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human K562 cells after 48 hrs by MTT assay, IC50 = 0.53 μM. | 28525838 | |
| BA/F3 | Function assay | 72 hrs | Inhibition of Kit exon 11 deletion (557 to 558 residues) and D816H mutant (unknown origin) transfected in mouse BA/F3 cells assessed as inhibition of cell growth incubated for 72 hrs by MTS assay, GI50 = 0.535 μM. | 30204441 | |
| BA/F3 | Function assay | 72 hrs | Inhibition of PDGFRalpha V561D/D842V mutant (unknown origin) transfected in mouse BA/F3 cells assessed as inhibition of cell growth incubated for 72 hrs by MTS assay, GI50 = 0.567 μM. | 30204441 | |
| K562 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human K562 cells after 48 hrs by MTT assay, IC50 = 0.58 μM. | 21295380 | |
| BA/F3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against mouse BA/F3 cells expressing BCR-ABL E359V mutant assessed as growth inhibition after 72 hrs by CCK-8 assay, IC50 = 0.59 μM. | 23301703 | |
| BA/F3 | Function assay | 72 hrs | Inhibition of BCR/ABL p210-M351T mutant (unknown origin) expressed in mouse BA/F3 cells assessed as growth inhibition after 72 hrs by CellTiter-Glo or CCK-8 assay, GI50 = 0.625 μM. | 26789553 | |
| BAF3 | Function assay | 72 hrs | Inhibition of BCR/ABL p210 fusion protein M356T mutant (unknown origin) expressed in BAF3 cells assessed as growth inhibition measured after 72 hrs by CellTiter-Glo or CCK8 assay, GI50 = 0.625 μM. | 27966954 | |
| BA/F3 | Function assay | 72 hrs | Inhibition of BCR/ABL p210-Q252H mutant (unknown origin) expressed in mouse BA/F3 cells assessed as growth inhibition after 72 hrs by CellTiter-Glo or CCK-8 assay, GI50 = 0.659 μM. | 26789553 | |
| BA/F3 | Function assay | 72 hrs | Inhibition of Kit exon 11 deletion (557 to 558 residues) and Y823D mutant (unknown origin) transfected in mouse BA/F3 cells assessed as inhibition of cell growth incubated for 72 hrs by MTS assay, GI50 = 0.669 μM. | 30204441 | |
| K562 | Apoptosis assay | 48 hrs | Induction of apoptosis in human K562 cells after 48 hrs by annexinV and propidium iodide staining based flow cytometry, AC50 = 0.68 μM. | 26629859 | |
| BA/F3 | Function assay | 72 hrs | Inhibition of Kit V560D/V654A mutant (unknown origin) transfected in mouse BA/F3 cells assessed as inhibition of cell growth incubated for 72 hrs by MTS assay, GI50 = 0.701 μM. | 30204441 | |
| K562 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human K562 cells assessed as cell viability after 72 hrs by fluorescence microplate reader method, IC50 = 0.73 μM. | 26741853 | |
| BAF3 | Function assay | 72 hrs | Inhibition of ABL in mouse BAF3 cells assessed as reduction in cell viability after 72 hrs by MTT assay, IC50 = 0.73 μM. | 28974338 | |
| insect cells | Function assay | 30 mins | Inhibition of human recombinant ABL1 Y253F mutant expressed in insect cells after 30 mins by FRET assay, IC50 = 0.7496 μM. | 23301703 | |
| K562 | Function assay | 2 hrs | Inhibition of BCR/ABL p210 autophosphorylation in human K562 cells after 2 hrs by Western blot analysis, IC50 = 0.75 μM. | 20188579 | |
| BAF3 | Function assay | 72 hrs | Inhibition of TEL-fused cKIT V559D/V654A double mutant (unknown origin) expressed in mouse BAF3 cells assessed as cell growth inhibition after 72 hrs by cell titer-glo assay, GI50 = 0.803 μM. | 28541695 | |
| BAF3 | Function assay | 72 hrs | Inhibition of TEL-fused cKIT V654A mutant (unknown origin) expressed in mouse BAF3 cells assessed as cell growth inhibition after 72 hrs by cell titer-glo assay, GI50 = 0.8031 μM. | 28541695 | |
| Ba/F3 | Function assay | 48 hrs | Inhibition of BCR-ABL1 Y253H mutant (unknown origin) expressed in mouse Ba/F3 cells assessed as inhibition of BCR-ABL1-mediated cell proliferation after 48 hrs by BriteLight luciferase assay, GI50 = 0.836 μM. | 30137981 | |
| Ba/F3 | Function assay | 48 hrs | Inhibition of BCR-ABL1 E255K mutant (unknown origin) expressed in mouse Ba/F3 cells assessed as inhibition of BCR-ABL1-mediated cell proliferation after 48 hrs by BriteLight luciferase assay, GI50 = 0.838 μM. | 30137981 | |
| BAF3 | Function assay | 72 hrs | Inhibition of BCR/ABL p210 fusion protein F317I mutant (unknown origin) expressed in BAF3 cells assessed as growth inhibition measured after 72 hrs by CellTiter-Glo or CCK8 assay, GI50 = 0.85 μM. | 27966954 | |
| BA/F3 | Function assay | 72 hrs | Inhibition of BCR/ABL p210-F317I mutant (unknown origin) expressed in mouse BA/F3 cells assessed as growth inhibition after 72 hrs by CellTiter-Glo or CCK-8 assay, GI50 = 0.855 μM. | 26789553 | |
| Ba/F3 | Function assay | 48 hrs | Inhibition of BCR-ABL1 E255V mutant (unknown origin) expressed in mouse Ba/F3 cells assessed as inhibition of BCR-ABL1-mediated cell proliferation after 48 hrs by BriteLight luciferase assay, GI50 = 0.874 μM. | 30137981 | |
| BA/F3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against mouse BA/F3 cells expressing Bcr-Abl F359V mutant after 72 hrs by CCK-8 assay, IC50 = 0.96 μM. | 23088644 | |
| BA/F3 | Antiproliferative assay | 48 hrs | Antiproliferation activity against mouse BA/F3 cells expressing Bcr-abl A334l mutant assessed as cell viability after 48 hrs by MTT assay, IC50 = 0.962 μM. | 16415863 | |
| K562 | Cytotoxicity assay | 24 hrs | Cytotoxicity against imatinib-sensitive human K562 cells assessed as decrease in cell viability after 24 hrs by XTT assay, IC50 = 1 μM. | 30261468 | |
| BA/F3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against mouse BA/F3 cells expressing BCR-ABL F486S mutant assessed as growth inhibition after 72 hrs by CCK-8 assay, IC50 = 1 μM. | 23301703 | |
| GIST430 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human GIST430 cells harboring KIT V654A mutant assessed as cell growth inhibition after 72 hrs by CellTiterGlo assay, GI50 = 1.1 μM. | 28991465 | |
| K562 | Cytotoxicity assay | 48 hrs | Cytotoxicity in drug sensitive human K562 cells assessed as reduction cell viability incubated for 48 hrs by XTT assay, IC50 = 1.1 μM. | 29655981 | |
| K562 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human K562 cells after 48 hrs by MTT assay, IC50 = 1.16 μM. | 26231079 | |
| K562 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human K562 cells expressing Bcr-Abl assessed as growth inhibition after 48 hrs by MTT assay, IC50 = 1.16 μM. | 26451772 | |
| BA/F3 | Function assay | 72 hrs | Inhibition of Kit V560D/D816H mutant (unknown origin) transfected in mouse BA/F3 cells assessed as inhibition of cell growth incubated for 72 hrs by MTS assay, GI50 = 1.191 μM. | 30204441 | |
| BAF3 | Function assay | 72 hrs | Inhibition of TEL-fused cKIT D816H mutant (unknown origin) expressed in mouse BAF3 cells assessed as cell growth inhibition after 72 hrs by cell titer-glo assay, GI50 = 1.245 μM. | 28541695 | |
| FDC-P1 | Function assay | 48 hrs | Inhibition of human FMS expressed in growth factor dependent mouse FDC-P1 cells assessed as inhibition of FMS-mediated cell proliferation in presence human CSF1 after 48 hrs by resazurin dye reduction assay, IC50 = 1.274 μM. | 20156689 | |
| BAF3 | Function assay | 72 hrs | Inhibition of Tel-fused cKIT N822K mutant (unknown origin) transfected in mouse BAF3 cells assessed as decrease in cell proliferation after 72 hrs by celltiter-glo/CCK8 assay, GI50 = 1.29 μM. | 27077705 | |
| BAF3 | Function assay | 72 hrs | Inhibition of Tel-fused c-KIT N822K mutant (unknown origin) expressed in BAF3 cells assessed as growth inhibition measured after 72 hrs by CellTiter-Glo or CCK8 assay, GI50 = 1.29 μM. | 27966954 | |
| BA/F3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against mouse BA/F3 cells expressing Bcr-Abl Y253F mutant after 72 hrs by CCK-8 assay, IC50 = 1.444 μM. | 23088644 | |
| BAF3 | Function assay | 72 hrs | Inhibition of TEL-fused cKIT N822K mutant (unknown origin) expressed in mouse BAF3 cells assessed as cell growth inhibition after 72 hrs by cell titer-glo assay, GI50 = 1.466 μM. | 28541695 | |
| BA/F3 | Function assay | 72 hrs | Inhibition of Kit exon 11 deletion (560 to 578 residues) mutant (unknown origin) transfected in mouse BA/F3 cells assessed as inhibition of cell growth incubated for 72 hrs by MTS assay, GI50 = 1.523 μM. | 30204441 | |
| BA/F3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against mouse BA/F3 cells expressing BCR-ABL E355G mutant assessed as growth inhibition after 72 hrs by CCK-8 assay, IC50 = 1.6 μM. | 23301703 | |
| BA/F3 | Function assay | 72 hrs | Inhibition of BCR/ABL p210-H369P mutant (unknown origin) expressed in mouse BA/F3 cells assessed as growth inhibition after 72 hrs by CellTiter-Glo or CCK-8 assay, GI50 = 1.69 μM. | 26789553 | |
| GIST882 | Antiproliferative assay | 96 hrs | Antiproliferative activity against human GIST882 cells after 96 hrs by SRB assay, IC50 = 1.7 μM. | 19469547 | |
| GIST882 | Antiproliferative assay | 96 hrs | Antiproliferative activity against imatinib-susceptible human GIST882 cells harboring c-KIT K642E mutant after 96 hrs by SRB assay, IC50 = 1.7 μM. | 29724653 | |
| BAF3 | Function assay | 72 hrs | Inhibition of BCR/ABL p210 fusion protein H369P mutant (unknown origin) expressed in BAF3 cells assessed as growth inhibition measured after 72 hrs by CellTiter-Glo or CCK8 assay, GI50 = 1.79 μM. | 27966954 | |
| BA/F3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against mouse BA/F3 cells expressing wild type Bcr-Abl after 72 hrs by CCK-8 assay, IC50 = 1.834 μM. | 23088644 | |
| BA/F3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against mouse BA/F3 cells expressing Bcr-Abl G250E mutant after 72 hrs by CCK-8 assay, IC50 = 1.863 μM. | 23088644 | |
| BA/F3 | Function assay | 72 hrs | Inhibition of BCR/ABL p210-E255K mutant (unknown origin) expressed in mouse BA/F3 cells assessed as growth inhibition after 72 hrs by CellTiter-Glo or CCK-8 assay, GI50 = 1.93 μM. | 26789553 | |
| BA/F3 | Function assay | 72 hrs | Inhibition of TEL-SRC (unknown origin) expressed in mouse BA/F3 cells assessed as growth inhibition after 72 hrs by CellTiter-Glo or CCK-8 assay, GI50 = 2.1 μM. | 26789553 | |
| NCI-H1703 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human NCI-H1703 cells after 72 hrs by CellTiter-Glo or CCK-8 assay, GI50 = 2.1 μM. | 29544149 | |
| BAF3 | Function assay | 72 hrs | Inhibition of BCR/ABL p210 fusion protein F317L mutant (unknown origin) expressed in BAF3 cells assessed as growth inhibition measured after 72 hrs by CellTiter-Glo or CCK8 assay, GI50 = 2.16 μM. | 27966954 | |
| BA/F3 | Function assay | 72 hrs | Inhibition of BCR/ABL p210-F317L mutant (unknown origin) expressed in mouse BA/F3 cells assessed as growth inhibition after 72 hrs by CellTiter-Glo or CCK-8 assay, GI50 = 2.169 μM. | 26789553 | |
| BA/F3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against mouse BA/F3 cells expressing Bcr-Abl H396R mutant after 72 hrs by CCK-8 assay, IC50 = 2.377 μM. | 23088644 | |
| HGC27 | Antiproliferative assay | 96 hrs | Antiproliferative activity against human HGC27 cells after 96 hrs by SRB assay, IC50 = 2.4 μM. | 19469547 | |
| HGC27 | Antiproliferative assay | 96 hrs | Antiproliferative activity against human HGC27 cells after 96 hrs by SRB assay, IC50 = 2.4 μM. | 29724653 | |
| BAF3 | Function assay | 72 hrs | Inhibition of Tel-fused cKIT V654A mutant (unknown origin) transfected in mouse BAF3 cells assessed as decrease in cell proliferation after 72 hrs by celltiter-glo/CCK8 assay, GI50 = 2.49 μM. | 27077705 | |
| BAF3 | Function assay | 72 hrs | Inhibition of Tel-fused c-KIT V654A mutant (unknown origin) expressed in BAF3 cells assessed as growth inhibition measured after 72 hrs by CellTiter-Glo or CCK8 assay, GI50 = 2.49 μM. | 27966954 | |
| BA/F3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against mouse BA/F3 cells expressing Bcr-Abl F486S mutant after 72 hrs by CCK-8 assay, IC50 = 2.547 μM. | 23088644 | |
| BA/F3 | Function assay | 72 hrs | Inhibition of Kit exon 9 AY502 to 503 insertion and D816 mutant (unknown origin) transfected in mouse BA/F3 cells assessed as inhibition of cell growth incubated for 72 hrs by MTS assay, GI50 = 2.749 μM. | 30204441 | |
| HEK293 | Function assay | 1.5 mins | Inhibition of human MATE2K-mediated ASP+ uptake expressed in HEK293 cells after 1.5 mins by fluorescence assay, IC50 = 2.9 μM. | 23241029 | |
| BA/F3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against mouse BA/F3 cells expressing Bcr-Abl E255K mutant after 72 hrs by CCK-8 assay, IC50 = 2.951 μM. | 23088644 | |
| BA/F3 | Function assay | 72 hrs | Inhibition of TEL-DDR1 (unknown origin) expressed in mouse BA/F3 cells assessed as growth inhibition after 72 hrs by CellTiter-Glo or CCK-8 assay, GI50 = 3 μM. | 26789553 | |
| BAF3 | Function assay | 72 hrs | Inhibition of Tel-fused cKIT V559D/V654A double mutant (unknown origin) transfected in mouse BAF3 cells assessed as decrease in cell proliferation after 72 hrs by celltiter-glo/CCK8 assay, GI50 = 3 μM. | 27077705 | |
| BAF3 | Function assay | 72 hrs | Inhibition of Tel-fused c-KIT V559D/V654A double mutant (unknown origin) expressed in BAF3 cells assessed as growth inhibition measured after 72 hrs by CellTiter-Glo or CCK8 assay, GI50 = 3 μM. | 27966954 | |
| BA/F3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against mouse BA/F3 cells expressing Bcr-Abl T315I mutant after 72 hrs by CCK-8 assay, IC50 = 3.005 μM. | 23088644 | |
| BA/F3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against mouse BA/F3 cells expressing BCR-ABL F317L mutant assessed as growth inhibition after 72 hrs by CCK-8 assay, IC50 = 3.3 μM. | 23301703 | |
| K562 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human K562 cells after 72 hrs by MTT assay, IC50 = 3.43 μM. | 29684708 | |
| BA/F3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against mouse BA/F3 cells expressing BCR-ABL Q252H mutant assessed as growth inhibition after 72 hrs by CCK-8 assay, IC50 = 3.6 μM. | 23301703 | |
| BA/F3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against mouse BA/F3 cells expressing Bcr-Abl Q252H mutant after 72 hrs by CCK-8 assay, IC50 = 3.763 μM. | 23088644 | |
| HGC27 | Cytotoxicity assay | 48 hrs | Cytotoxicity against HGC27 cells assessed as cell viability after 48 hrs by MTT assay, IC50 = 3.8 μM. | 24900584 | |
| BA/F3 | Function assay | 72 hrs | Inhibition of Kit exon 9 AY502 to 503 insertion and V654 mutant (unknown origin) transfected in mouse BA/F3 cells assessed as inhibition of cell growth incubated for 72 hrs by MTS assay, GI50 = 3.947 μM. | 30204441 | |
| BA/F3 | Function assay | 72 hrs | Inhibition of TEL-BLK (unknown origin) expressed in mouse BA/F3 cells assessed as growth inhibition after 72 hrs by CellTiter-Glo or CCK-8 assay, GI50 = 4.1 μM. | 26789553 | |
| K562 | Antiproliferative assay | 48 hrs | Antiproliferative activity against Bcr/Abl positive human K562 cells after 48 hrs by MTT method, IC50 = 4.12 μM. | 25464886 | |
| K562 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human K562 cells after 48 hrs by MTT assay, IC50 = 4.12 μM. | 26298495 | |
| HEK293 | Function assay | 3 mins | Inhibition of human OCT2-mediated ASP+ uptake expressed in HEK293 cells after 3 mins by fluorescence assay, IC50 = 4.2 μM. | 23241029 | |
| A549 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human A549 cells after 48 hrs by MTT assay, IC50 = 4.56 μM. | 28525838 | |
| K562/G | Cytotoxicity assay | 72 hrs | Cytotoxicity against human K562/G cells assessed as cell viability after 72 hrs by MTT assay, IC50 = 4.65 μM. | 26814890 | |
| BAF3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against mouse BAF3 cells assessed as cell growth inhibition after 72 hrs by cell titer-glo assay, GI50 = 4.686 μM. | 28541695 | |
| BA/F3 | Cytotoxicity assay | 48 hrs | Cytotoxicity against mouse BA/F3 cells transfected with Bcr-Abl T315I mutant after 48 hrs by XTT assay, IC50 = 4.79 μM. | 23600806 | |
| insect cells | Function assay | 30 mins | Inhibition of human recombinant ABL1 T315I mutant expressed in insect cells after 30 mins by FRET assay, IC50 = 5.155 μM. | 23301703 | |
| BA/F3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against mouse BA/F3 cells expressing BCR-ABL G250E mutant assessed as growth inhibition after 72 hrs by CCK-8 assay, IC50 = 5.2 μM. | 23301703 | |
| HEL | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HEL cells assessed as cell viability after 72 hrs by CellTiter-Glo or CCK-8 assay, GI50 = 5.3 μM. | 26789553 | |
| K562 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human K562 cells after 48 hrs by MTT assay, IC50 = 5.4 μM. | 21576023 | |
| KBM5 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human KBM5 cells harboring Bcr-Abl T315I mutant after 72 hrs by MTT assay, IC50 = 5.4 μM. | 20149665 | |
| BA/F3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against mouse BA/F3 cells expressing BCR-ABL E255K mutant assessed as growth inhibition after 72 hrs by CCK-8 assay, IC50 = 5.6 μM. | 23301703 | |
| BAF3 | Function assay | 72 hrs | Inhibition of Tel-fused cKIT T670I/V559D double mutant (unknown origin) transfected in mouse BAF3 cells assessed as decrease in cell proliferation after 72 hrs by celltiter-glo/CCK8 assay, GI50 = 6.67 μM. | 27077705 | |
| BAF3 | Function assay | 72 hrs | Inhibition of Tel-fused c-KIT T670I mutant (unknown origin) expressed in BAF3 cells assessed as growth inhibition measured after 72 hrs by CellTiter-Glo or CCK8 assay, GI50 = 6.67 μM. | 27966954 | |
| BA/F3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against mouse BA/F3 cells assessed as cell viability after 72 hrs by CellTiter-Glo or CCK-8 assay, GI50 = 6.7 μM. | 26789553 | |
| GIST48B | Antiproliferative assay | 72 hrs | Antiproliferative activity against human GIST48B cells assessed as cell growth inhibition after 72 hrs by cell titer-glo assay, GI50 = 6.986 μM. | 28541695 | |
| MCF7 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MCF7 cells after 72 hrs by MTT assay, IC50 = 7.12 μM. | 29684708 | |
| BA/F3 | Function assay | 72 hrs | Inhibition of TEL-DDR2 (unknown origin) expressed in mouse BA/F3 cells assessed as growth inhibition after 72 hrs by CellTiter-Glo or CCK-8 assay, GI50 = 7.7 μM. | 26789553 | |
| FDC-P1 | Function assay | 48 hrs | Inhibition of mouse GM-CSF-stimulated cell proliferation of growth factor dependent mouse FDC-P1 cells expressing human FMS after 48 hrs by resazurin dye reduction assay, IC50 = 8.437 μM. | 20156689 | |
| BA/F3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against mouse BA/F3 cells expressing Bcr-Abl E255V mutant after 72 hrs by CCK-8 assay, IC50 = 8.615 μM. | 23088644 | |
| A2780 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human A2780 cells after 72 hrs by MTT assay, IC50 = 8.9 μM. | 29684708 | |
| BESM | Antitrypanosomal assay | 88 hrs | Antitrypanosomal activity against Trypanosoma cruzi amastigotes infected in BESM cells measured after 88 hrs postinfection by HTS assay, EC50 = 9 μM. | 20547819 | |
| BA/F3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against mouse BA/F3 cells expressing BCR-ABL Y253F mutant assessed as growth inhibition after 72 hrs by CCK-8 assay, IC50 = 9.2 μM. | 23301703 | |
| BA/F3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against mouse BA/F3 cells expressing BCR-ABL F317V mutant assessed as growth inhibition after 72 hrs by CCK-8 assay, IC50 = 9.5 μM. | 23301703 | |
| Ba/F3 | Function assay | 48 hrs | Inhibition of BCR-ABL1 T315I mutant (unknown origin) expressed in mouse Ba/F3 cells assessed as inhibition of cell proliferation after 48 hrs by BriteLight luciferase assay, GI50 = 9.645 μM. | 30137981 | |
| BA/F3 | Function assay | 72 hrs | Inhibition of TEL-HCK (unknown origin) expressed in mouse BA/F3 cells assessed as growth inhibition after 72 hrs by CellTiter-Glo or CCK-8 assay, GI50 = 9.7 μM. | 26789553 | |
| BA/F3 | Function assay | 72 hrs | Inhibition of Kit D816V mutant (unknown origin) transfected in mouse BA/F3 cells assessed as inhibition of cell growth incubated for 72 hrs by MTS assay, GI50 = 9.93 μM. | 30204441 | |
| HCT116 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT116 cells after 72 hrs by alamar-blue cell viability assay, IC50 = 10 μM. | 19301902 | |
| HepG2 | Antiproliferative assay | 24 to 96 hrs | Antiproliferative activity against human HepG2 cells after 24 to 96 hrs by MTS assay, IC50 = 10 μM. | 23932071 | |
| BA/F3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against mouse BA/F3 cells expressing BCR-ABL H396R mutant assessed as growth inhibition after 72 hrs by CCK-8 assay, IC50 = 11.1 μM. | 23301703 | |
| MCF7 | Antiproliferative assay | 96 hrs | Antiproliferative activity against human MCF7 cells after 96 hrs by SRB assay, IC50 = 11.3 μM. | 29724653 | |
| MCF7 | Antiproliferative assay | 96 hrs | Antiproliferative activity against human MCF7 cells after 96 hrs by SRB assay, IC50 = 11.5 μM. | 19469547 | |
| RKOp21 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human RKOp21 cells after 72 hrs by alamar-blue cell viability assay, IC50 = 12 μM. | 19301902 | |
| BA/F3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against mouse BA/F3 cells expressing BCR-ABL L248V mutant assessed as growth inhibition after 72 hrs by CCK-8 assay, IC50 = 12.2 μM. | 23301703 | |
| BA/F3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against mouse BA/F3 cells expressing BCR-ABL T315I mutant assessed as growth inhibition after 72 hrs by CCK-8 assay, IC50 = 12.2 μM. | 23301703 | |
| BA/F3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against mouse BA/F3 cells expressing BCR-ABL Y253H mutant assessed as growth inhibition after 72 hrs by CCK-8 assay, IC50 = 12.5 μM. | 23301703 | |
| RKOp21 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human RKOp21 cells after 72 hrs by alamar-blue cell viability assay, IC50 = 12.5892 μM. | 19301902 | |
| K562 | Antiproliferative assay | 24 hrs | In vitro antiproliferative activity against human K562 cells assessed as decrease in cell viability after 24 hrs by MTT assay, IC50 = 13 μM. | 24681986 | |
| BA/F3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against mouse BA/F3 cells assessed as growth inhibition after 72 hrs by CCK-8 assay, IC50 = 13.5 μM. | 23301703 | |
| BA/F3 | Growth inhibition assay | 48 hrs | Growth inhibition of mouse BA/F3 cells expressing Bcr-Abl T315I mutant after 48 hrs by MTT assay, IC50 = 14.5 μM. | 26562217 | |
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells after 72 hrs by alamar-blue cell viability assay, IC50 = 15.5 μM. | 19301902 | |
| K562 | Antiproliferative assay | 48 hrs | Antiproliferative activity against imatinib-resistant human K562 cells after 48 hrs by MTT assay, IC50 = 15.7 μM. | 26231079 | |
| MDA-MB-468 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MDA-MB-468 cells after 72 hrs by alamar-blue cell viability assay, IC50 = 15.8489 μM. | 19301902 | |
| KG1a | Antiproliferative assay | 72 hrs | Antiproliferative activity against human KG1a cells assessed as inhibition of cell survival after 72 hrs by MTT assay, IC50 = 16.7 μM. | 26850004 | |
| MDA-MB-468 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MDA-MB-468 cells after 72 hrs by alamar-blue cell viability assay, IC50 = 17 μM. | 19301902 | |
| BA/F3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against mouse BA/F3 cells expressing Bcr-Abl Y253H mutant after 72 hrs by CCK-8 assay, IC50 = 17.083 μM. | 23088644 | |
| BA/F3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against mouse BA/F3 cells after 72 hrs by CCK-8 assay, IC50 = 18.52 μM. | 23088644 | |
| HeLa | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HeLa cells after 72 hrs by alamar-blue cell viability assay, IC50 = 18.65 μM. | 19301902 | |
| GIST48 | Cytotoxicity assay | 3 to 6 days | Cytotoxicity against human GIST48 cells assessed as effect on cell viability after 3 to 6 days by luciferase based luminescence assay, IC50 = 18.7 μM. | 19469547 | |
| Hec1A | Cytotoxicity assay | 72 hrs | Cytotoxicity against human Hec1A cells after 72 hrs by alamar-blue cell viability assay, IC50 = 19 μM. | 19301902 | |
| HCT116 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT116 cells after 72 hrs by MTT assay, IC50 = 19.66 μM. | 29684708 | |
| GIST48 | Antiproliferative assay | 96 hrs | Antiproliferative activity against imatinib-resistant human GIST48 cells harboring c-KIT V560D/D820A double mutant after 96 hrs by SRB assay, IC50 = 19.8 μM. | 29724653 | |
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells after 72 hrs by alamar-blue cell viability assay, IC50 = 19.9526 μM. | 19301902 | |
| CAL27 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human CAL27 cells after 72 hrs by alamar-blue cell viability assay, IC50 = 19.9526 μM. | 19301902 | |
| Hec1A | Cytotoxicity assay | 72 hrs | Cytotoxicity against human Hec1A cells after 72 hrs by alamar-blue cell viability assay, IC50 = 19.9526 μM. | 19301902 | |
| GIST48B | Growth inhibition assay | 72 hrs | Growth inhibition of human GIST48B cells after 72 hrs by SRB assay, GI50 = 20 μM. | 23773153 | |
| BV173 | Antiproliferative assay | 24 hrs | In vitro antiproliferative activity against human BV173 cells assessed as decrease in cell viability after 24 hrs by MTT assay, IC50 = 20 μM. | 24681986 | |
| BAF3 | Function assay | 72 hrs | Inhibition of ABL T315I mutant in mouse BAF3 cells assessed as reduction in cell viability after 72 hrs by MTT assay, IC50 = 20.03 μM. | 28974338 | |
| CAL27 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human CAL27 cells after 72 hrs by alamar-blue cell viability assay, IC50 = 22 μM. | 19301902 | |
| H460 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human H460 cells after 72 hrs by alamar-blue cell viability assay, IC50 = 24 μM. | 19301902 | |
| PC3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human PC3 cells after 72 hrs by alamar-blue cell viability assay, IC50 = 24 μM. | 19301902 | |
| HCT15 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT15 cells after 72 hrs by alamar-blue cell viability assay, IC50 = 25 μM. | 19301902 | |
| HeLa | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HeLa cells after 72 hrs by alamar-blue cell viability assay, IC50 = 25.1189 μM. | 19301902 | |
| H460 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human H460 cells after 72 hrs by alamar-blue cell viability assay, IC50 = 25.1189 μM. | 19301902 | |
| HCT15 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT15 cells after 72 hrs by alamar-blue cell viability assay, IC50 = 25.1189 μM. | 19301902 | |
| PC3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human PC3 cells after 72 hrs by alamar-blue cell viability assay, IC50 = 25.1189 μM. | 19301902 | |
| Saos2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human Saos2 cells after 72 hrs by alamar-blue cell viability assay, IC50 = 25.1189 μM. | 19301902 | |
| HEK293 | Function assay | 3 mins | Inhibition of human OCT3-mediated ASP+ uptake expressed in HEK293 cells after 3 mins by fluorescence assay, IC50 = 25.5 μM. | 23241029 | |
| Saos2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human Saos2 cells after 72 hrs by alamar-blue cell viability assay, IC50 = 26 μM. | 19301902 | |
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by alamar-blue cell viability assay, IC50 = 30 μM. | 19301902 | |
| A431 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A431 cells after 72 hrs by alamar-blue cell viability assay, IC50 = 31 μM. | 19301902 | |
| H69 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human H69 cells after 72 hrs by alamar-blue cell viability assay, IC50 = 31.6228 μM. | 19301902 | |
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by alamar-blue cell viability assay, IC50 = 31.6228 μM. | 19301902 | |
| A431 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A431 cells after 72 hrs by alamar-blue cell viability assay, IC50 = 31.6228 μM. | 19301902 | |
| H69 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human H69 cells after 72 hrs by alamar-blue cell viability assay, IC50 = 32 μM. | 19301902 | |
| BESM | Cytotoxicity assay | 88 hrs | Cytotoxicity against BESM cells after 88 hrs by HTS assay, EC50 = 32 μM. | 20547819 | |
| CCRF-CEM | Cytotoxicity assay | 72 hrs | Cytotoxicity against human CCRF-CEM cells after 72 hrs by alamar-blue cell viability assay, IC50 = 36 μM. | 19301902 | |
| MDA-MB-231 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MDA-MB-231 cells after 72 hrs by alamar-blue cell viability assay, IC50 = 37 μM. | 19301902 | |
| CCRF-CEM/VCR1000 | Cytotoxicity assay | 72 hrs | Cytotoxicity against vincristine-resistant human CCRF-CEM/VCR1000 cells after 72 hrs by alamar-blue cell viability assay, IC50 = 37 μM. | 19301902 | |
| WM266.4 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human WM266.4 cells after 72 hrs by alamar-blue cell viability assay, IC50 = 37 μM. | 19301902 | |
| U87MG | Cytotoxicity assay | 72 hrs | Cytotoxicity against human U87MG cells after 72 hrs by alamar-blue cell viability assay, IC50 = 38 μM. | 19301902 | |
| SKBR3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human SKBR3 cells after 72 hrs by alamar-blue cell viability assay, IC50 = 39 μM. | 19301902 | |
| MDA-MB-231 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MDA-MB-231 cells after 72 hrs by alamar-blue cell viability assay, IC50 = 39.8107 μM. | 19301902 | |
| SKBR3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human SKBR3 cells after 72 hrs by alamar-blue cell viability assay, IC50 = 39.8107 μM. | 19301902 | |
| AsPC1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human AsPC1 cells after 72 hrs by alamar-blue cell viability assay, IC50 = 39.8107 μM. | 19301902 | |
| CCRF-CEM/VCR1000 | Cytotoxicity assay | 72 hrs | Cytotoxicity against vincristine-resistant human CCRF-CEM/VCR1000 cells after 72 hrs by alamar-blue cell viability assay, IC50 = 39.8107 μM. | 19301902 | |
| CCRF-CEM | Cytotoxicity assay | 72 hrs | Cytotoxicity against human CCRF-CEM cells after 72 hrs by alamar-blue cell viability assay, IC50 = 39.8107 μM. | 19301902 | |
| U87MG | Cytotoxicity assay | 72 hrs | Cytotoxicity against human U87MG cells after 72 hrs by alamar-blue cell viability assay, IC50 = 39.8107 μM. | 19301902 | |
| WM266.4 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human WM266.4 cells after 72 hrs by alamar-blue cell viability assay, IC50 = 39.8107 μM. | 19301902 | |
| AsPC1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human AsPC1 cells after 72 hrs by alamar-blue cell viability assay, IC50 = 44 μM. | 19301902 | |
| CCRF-CEM | Antiproliferative assay | 24 hrs | In vitro antiproliferative activity against human CCRF-CEM cells assessed as decrease in cell viability after 24 hrs by MTT assay, IC50 = 45 μM. | 24681986 | |
| A2780 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A2780 cells after 72 hrs by alamar-blue cell viability assay, IC50 = 48 μM. | 19301902 | |
| Vero | Antiviral assay | 3 days | Antiviral activity against Dengue virus infected in african green monkey Vero cells administered before viral challenge after 3 days by viral plaque assay | 17360676 | |
| Ba/F3 | Function assay | 1 hr | Inhibition of Bcr-Abl T315I mutant (unknown origin) transfected in mouse Ba/F3 cells assessed as reduction of phosphorylated STAT5 level after 1 hr by Western blot analysis | 23600806 | |
| Ba/F3 | Function assay | 1 hr | Inhibition of Bcr-Abl T315I mutant (unknown origin) transfected in mouse Ba/F3 cells assessed as reduction of phosphorylated CrkL level after 1 hr by Western blot analysis | 23600806 | |
| Ba/F3 | Function assay | 1 hr | Inhibition of Bcr-Abl T315I mutant (unknown origin) phosphorylation transfected in mouse Ba/F3 cells after 1 hr by Western blot analysis | 23600806 | |
| K562 | Function assay | 6 hrs | Inhibition of BCR-ABL-mediated STAT5 phosphorylation in human K562 cells after 6 hrs by phospho-flow cytometry | 22148584 | |
| K-562 | Growth Inhibition Assay | IC50=1 μM | 25239662 | ||
| K562 | Growth Inhibition Assay | EC50=0.09 μM | 16678414 | ||
| K562r | Growth Inhibition Assay | IC50=10 μM | 24939418 | ||
| K562 | Growth Inhibition Assay | IC50=0.5 μM | 24939418 | ||
| Hep G2 | Growth Inhibition Assay | IC50=31 μM | 25863232 | ||
| T47D | Growth Inhibition Assay | IC50=50 μM | 25863232 | ||
| Sf9 | Function assay | Binding affinity to human active site of N-terminal hexahistidine-tagged ABL2 expressed in Trichoplusia ni infected Sf9 cells by isothermal titration calorimetric assay, Kd = 0.006 μM. | 21417343 | ||
| K562 | Antiproliferative assay | Antiproliferative activity against human imatinib-resistant K562 cells, IC50 = 0.00605 μM. | 21376587 | ||
| TF1 | Cytotoxicity assay | Cytotoxicity against human TF1 cells expressing c-KIT mutation assessed as cell viability, IC50 = 0.013 μM. | 22221201 | ||
| BA/F3 | Antiproliferative assay | Antiproliferative activity against PDGFRbeta transfected mouse BA/F3 cells, IC50 = 0.039 μM. | 20817538 | ||
| HEK293 | Function assay | Inhibition of autophosphorylation of DDR1 expressed in HEK293 cells by ELISA, IC50 = 0.043 μM. | 20817538 | ||
| MO7e | Function assay | Inhibition of SCF-induced phosphorylation of c-Kit in MO7e cells by TR-FRET assay, IC50 = 0.046 μM. | 18447379 | ||
| K562 | Cytotoxicity assay | Cytotoxicity against human K562 cells assessed as reduction in cell viability, IC50 = 0.06 μM. | 26264503 | ||
| A31 | Function assay | Inhibition of PDGFRbeta autophosphorylation in human A31 cells by ELISA, IC50 = 0.072 μM. | 20817538 | ||
| A31 | Function assay | Inhibition of human PDGFRalpha autophosphorylation in human A31 cells by ELISA, IC50 = 0.072 μM. | 20817538 | ||
| GIST882 | Function assay | Inhibition of human KIT autophosphorylation in human GIST882 cells by ELISA, IC50 = 0.097 μM. | 20817538 | ||
| GIST882 | Function assay | Inhibition of KIT K642E mutant autophosphorylation (unknown origin) expressed in human GIST882 cells by ELISA, IC50 = 0.097 μM. | 23611771 | ||
| GIST882 | Antiproliferative assay | Antiproliferative activity against human GIST882 cells, IC50 = 0.108 μM. | 20817538 | ||
| GIST882 | Function assay | Inhibition of KIT K642E mutant (unknown origin) expressed in human GIST882 cells assessed as cell growth inhibition by ATP-depletion assay, IC50 = 0.108 μM. | 23611771 | ||
| K562 | Cytotoxicity assay | Cytotoxic effect in K562 cells, IC50 = 0.11 μM. | 12951113 | ||
| HEK293 | Function assay | Inhibition of autophosphorylation of DDR2 expressed in HEK293 cells by ELISA, IC50 = 0.141 μM. | 20817538 | ||
| K562 | Cytotoxicity assay | Cytotoxicity against human K562 cells, IC50 = 0.17 μM. | 23600806 | ||
| K562 | Antiproliferative assay | Antiproliferative activity against human K562 cells, GI50 = 0.17 μM. | 22439674 | ||
| K562 | Cytotoxicity assay | Cytotoxicity against K562 cells by MTT assay, IC50 = 0.18 μM. | 17572088 | ||
| K562 | Antiproliferative assay | Antiproliferative activity against K562 cells, IC50 = 0.182 μM. | 16332440 | ||
| 32D | Cytotoxicity assay | Cytotoxic activity against mouse 32D cells transfected with p210 Bcr-abl, IC50 = 0.19 μM. | 16415863 | ||
| 32D | Function assay | Inhibition of human BCR-ABL1 (unknown origin) expressed in mouse 32D cells, IC50 = 0.194 μM. | 23611771 | ||
| Sf9 | Function assay | Inhibition of human Lyn kinase expressed in Sf9 cells, IC50 = 0.22 μM. | 17376680 | ||
| Ba/F | Function assay | Inhibition of autophosphorylation of BCR-ABL1 expressed in Ba/F cells, IC50 = 0.221 μM. | 20817538 | ||
| BA/F3 | Function assay | Inhibition of human BCR-ABL1 autophosphorylation expressed in mouse BA/F3 cells by ELISA, IC50 = 0.221 μM. | 23611771 | ||
| CHO | Function assay | Inhibition of wild type Platelet-derived growth factor receptor beta phosphorylation in CHO cells, IC50 = 0.24 μM. | 12166950 | ||
| K562 | Antiproliferative assay | Antiproliferative activity against human K562 cells, IC50 = 0.244 μM. | 20817538 | ||
| CHO | Function assay | Inhibition of chimeric PDGF receptor with c-kit cytoplasmic domain phosphorylation in CHO cells, IC50 = 0.26 μM. | 12166950 | ||
| HEK293 | Function assay | Inhibition of autophosphorylation of CSF1R expressed in HEK293 cells by ELISA, IC50 = 0.291 μM. | 20817538 | ||
| Sf9 | Function assay | Inhibition of recombinant Abl kinase (unknown origin) expressed in Sf9 cells using GGEAIYAAPFKK as substrate in presence of [gamma33P]ATP, IC50 = 0.3 μM. | 24681986 | ||
| ALL3 | Cytotoxicity assay | Cytotoxicity against Philadelphia chromosome positive human ALL3 cells by Alamar Blue fluorescent assay, IC50 = 0.333 μM. | 19889540 | ||
| KU812 | Antiproliferative assay | Antiproliferative activity against human KU812 cells, IC50 = 0.337 μM. | 21376587 | ||
| CML | Antiproliferative assay | Antiproliferative activity against human CML cells, IC50 = 0.35 μM. | 19219016 | ||
| M-NFS-60 | Antiproliferative assay | Antiproliferative activity against mouse M-NFS-60 cells, IC50 = 0.358 μM. | 20817538 | ||
| BAF3 | Function assay | Inhibition of Tel-fused c-KIT (unknown origin) expressed in BAF3 cells assessed as growth inhibition measured after 72 hrs by CellTiter-Glo or CCK8 assay, GI50 = 0.37 μM. | 27966954 | ||
| K562 | Antiproliferative assay | Antiproliferative activity against human K562 cells, IC50 = 0.38 μM. | 21376587 | ||
| K562 | Function assay | Inhibitory activity against human K562 cells growth using MTT assay, IC50 = 0.4 μM. | 14552760 | ||
| Sf9 | Function assay | Inhibition of human Abl kinase expressed in Sf9 cells, IC50 = 0.47 μM. | 17376680 | ||
| K562 | Function assay | Inhibition of BCR-ABL1 autophosphorylation in human K562 cells, IC50 = 0.473 μM. | 20817538 | ||
| Sf9 | Function assay | Binding affinity to human remote site of N-terminal hexahistidine-tagged ABL2 expressed in Trichoplusia ni infected Sf9 cells by isothermal titration calorimetric assay, Kd = 0.5 μM. | 21417343 | ||
| K562 | Antiproliferative assay | Antiproliferative activity against human K562 cells, IC50 = 0.5 μM. | 23352483 | ||
| K562 | Cytotoxicity assay | Cytotoxicity against human K562 cells, IC50 = 0.5 μM. | 27189674 | ||
| Sf9 | Function assay | TP_TRANSPORTER: inhibition of ATPase activity in BCRP-expressing Sf9 cells, IC50 = 0.5 μM. | 15155841 | ||
| BA/F3 | Antiproliferative assay | Antiproliferative activity against BCR-ABL1 transfected mouse BA/F3 cells, IC50 = 0.678 μM. | 20817538 | ||
| BA/F3 | Function assay | Inhibition of human BCR-ABL1 expressed in mouse BA/F3 cells assessed as cell growth inhibition by ATP-depletion assay, IC50 = 0.678 μM. | 23611771 | ||
| Sf9 | Function assay | TP_TRANSPORTER: efflux of Hoechst33342 in BCRP-expressing Sf9 cells, IC50 = 0.9 μM. | 15155841 | ||
| CHO | Function assay | Inhibition of chimeric PDGF receptor with CSF-1R cytoplasmic domain phosphorylation in CHO cells, IC50 = 0.96 μM. | 12166950 | ||
| IR-K562 | Cytotoxicity assay | Cytotoxicity against imatinib resistant human IR-K562 cells assessed as reduction in cell viability, IC50 = 1.1 μM. | 26264503 | ||
| MDCK | Function assay | Inhibition of BCRP expressed in MDCK cells by pheophorbide A assay, IC50 = 3.38 μM. | 19932960 | ||
| MCF7 MX | Function assay | Inhibition of BCRP expressed in MCF7 MX cells by Hoechst 33342 staining, IC50 = 4.07 μM. | 19932960 | ||
| MCF7 | Function assay | Inhibition of ABCG2 in human mitoxantrone-resistant MCF7 cells by Hoechst 33342 assay, IC50 = 4.89779 μM. | 18678495 | ||
| Sf9 | Function assay | Binding affinity to recombinant full length human N-terminal GST-tagged Syk-KD (356 to 635 residues) cytoplasmic domain expressed in baculovirus infected Sf9 insect cells using poly EY 4:1 as substrate, Ki = 5 μM. | 29132752 | ||
| BaF3 | Antiproliferative assay | Antiproliferative activity against murine BaF3 cells expressing wild type NPM/ALK by [3H]thymidine uptake assay, IC50 = 7.1 μM. | 16970400 | ||
| A2780 | Function assay | Inhibition of P-gp in human adriamycin-resistant A2780 cells by Hoechst 33342 assay, IC50 = 7.24436 μM. | 18678495 | ||
| A2780/ADR | Function assay | Inhibition of P-glycoprotein expressed in A2780/ADR cells by calcein AM assay, IC50 = 7.24436 μM. | 17890094 | ||
| HEK293 | Cytotoxicity assay | Cytotoxicity against HEK293 cells assessed as reduction in cell viability, CC50 = 8.4 μM. | 26264503 | ||
| BaF3 | Antiproliferative assay | Antiproliferative activity against murine BaF3 cells expressing NPM/ALK L256T mutant by [3H]thymidine uptake assay, IC50 = 9.1 μM. | 16970400 | ||
| BA/F3 | Antiproliferative assay | Antiproliferative activity mouse BA/F3 cells in presence of interleukin 3, IC50 = 9.459 μM. | 20817538 | ||
| U937 | Antiproliferative assay | Antiproliferative activity against U937 cells, IC50 = 14 μM. | 16332440 | ||
| HMC-1.2 | Antiproliferative assay | Antiproliferative activity against human HMC-1.2 cells carrying V560G, D816V mutant assessed as cell growth inhibition by MTT assay, IC50 = 17 μM. | 25004409 | ||
| HUVEC | Antiproliferative assay | Antiproliferative activity against HUVEC cells, GI50 = 18.5 μM. | 22439674 | ||
| U937 | Cytotoxicity assay | Cytotoxicity against human U937 cells assessed as reduction in cell viability, IC50 = 19 μM. | 26264503 | ||
| 32D | Cytotoxicity assay | Cytotoxic activity against mouse 32D Bcr-abl negative cells, IC50 = 20 μM. | 16415863 | ||
| 293T | Cytotoxicity assay | Cytotoxicity against human 293T cells assessed as growth inhibition, CC50 = 20.53 μM. | 26850004 | ||
| THP1 | Cytotoxicity assay | Cytotoxicity against human THP1 cells assessed as reduction in cell viability, IC50 = 25 μM. | 26264503 | ||
| HCT116 | Anticancer assay | Anticancer activity against human HCT116 cells, IC50 = 34.4 μM. | 26312434 | ||
| DAOY | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for DAOY cells | 29435139 | ||
| LAMA-84 | Growth Inhibition assay | Inhibition of human LAMA-84 cell growth in a cell viability assay, IC50 = 0.07304 μM. | SANGER | ||
| EM-2 | Growth Inhibition assay | Inhibition of human EM-2 cell growth in a cell viability assay, IC50 = 0.0888 μM. | SANGER | ||
| MEG-01 | Growth Inhibition assay | Inhibition of human MEG-01 cell growth in a cell viability assay, IC50 = 0.08921 μM. | SANGER | ||
| BV-173 | Growth Inhibition assay | Inhibition of human BV-173 cell growth in a cell viability assay, IC50 = 0.1874 μM. | SANGER | ||
| K-562 | Growth Inhibition assay | Inhibition of human K-562 cell growth in a cell viability assay, IC50 = 0.22432 μM. | SANGER | ||
| CGTH-W-1 | Growth Inhibition assay | Inhibition of human CGTH-W-1 cell growth in a cell viability assay, IC50 = 0.38374 μM. | SANGER | ||
| ST486 | Growth Inhibition assay | Inhibition of human ST486 cell growth in a cell viability assay, IC50 = 0.6854 μM. | SANGER | ||
| NCI-H1436 | Growth Inhibition assay | Inhibition of human NCI-H1436 cell growth in a cell viability assay, IC50 = 0.97801 μM. | SANGER | ||
| NOS-1 | Growth Inhibition assay | Inhibition of human NOS-1 cell growth in a cell viability assay, IC50 = 1.65383 μM. | SANGER | ||
| A498 | Growth Inhibition assay | Inhibition of human A498 cell growth in a cell viability assay, IC50 = 2.57223 μM. | SANGER | ||
| BE-13 | Growth Inhibition assay | Inhibition of human BE-13 cell growth in a cell viability assay, IC50 = 2.62106 μM. | SANGER | ||
| SUP-T1 | Growth Inhibition assay | Inhibition of human SUP-T1 cell growth in a cell viability assay, IC50 = 3.82907 μM. | SANGER | ||
| NCI-H1770 | Growth Inhibition assay | Inhibition of human NCI-H1770 cell growth in a cell viability assay, IC50 = 5.57262 μM. | SANGER | ||
| IMR-5 | Growth Inhibition assay | Inhibition of human IMR-5 cell growth in a cell viability assay, IC50 = 6.22147 μM. | SANGER | ||
| LB2241-RCC | Growth Inhibition assay | Inhibition of human LB2241-RCC cell growth in a cell viability assay, IC50 = 8.07384 μM. | SANGER | ||
| TGBC24TKB | Growth Inhibition assay | Inhibition of human TGBC24TKB cell growth in a cell viability assay, IC50 = 8.34052 μM. | SANGER | ||
| SCC-15 | Growth Inhibition assay | Inhibition of human SCC-15 cell growth in a cell viability assay, IC50 = 10.7788 μM. | SANGER | ||
| BB49-HNC | Growth Inhibition assay | Inhibition of human BB49-HNC cell growth in a cell viability assay, IC50 = 14.3335 μM. | SANGER | ||
| ES7 | Growth Inhibition assay | Inhibition of human ES7 cell growth in a cell viability assay, IC50 = 14.7379 μM. | SANGER | ||
| LB2518-MEL | Growth Inhibition assay | Inhibition of human LB2518-MEL cell growth in a cell viability assay, IC50 = 16.6094 μM. | SANGER | ||
| NCI-H510A | Growth Inhibition assay | Inhibition of human NCI-H510A cell growth in a cell viability assay, IC50 = 17.2442 μM. | SANGER | ||
| TE-441-T | Growth Inhibition assay | Inhibition of human TE-441-T cell growth in a cell viability assay, IC50 = 17.2886 μM. | SANGER | ||
| HH | Growth Inhibition assay | Inhibition of human HH cell growth in a cell viability assay, IC50 = 17.3999 μM. | SANGER | ||
| LC4-1 | Growth Inhibition assay | Inhibition of human LC4-1 cell growth in a cell viability assay, IC50 = 18.0652 μM. | SANGER | ||
| KARPAS-45 | Growth Inhibition assay | Inhibition of human KARPAS-45 cell growth in a cell viability assay, IC50 = 18.1848 μM. | SANGER | ||
| LB1047-RCC | Growth Inhibition assay | Inhibition of human LB1047-RCC cell growth in a cell viability assay, IC50 = 18.4452 μM. | SANGER | ||
| NKM-1 | Growth Inhibition assay | Inhibition of human NKM-1 cell growth in a cell viability assay, IC50 = 19.3552 μM. | SANGER | ||
| SCLC-21H | Growth Inhibition assay | Inhibition of human SCLC-21H cell growth in a cell viability assay, IC50 = 20.1246 μM. | SANGER | ||
| RS4-11 | Growth Inhibition assay | Inhibition of human RS4-11 cell growth in a cell viability assay, IC50 = 20.3308 μM. | SANGER | ||
| ALL-PO | Growth Inhibition assay | Inhibition of human ALL-PO cell growth in a cell viability assay, IC50 = 20.8149 μM. | SANGER | ||
| GDM-1 | Growth Inhibition assay | Inhibition of human GDM-1 cell growth in a cell viability assay, IC50 = 22.5945 μM. | SANGER | ||
| DMS-79 | Growth Inhibition assay | Inhibition of human DMS-79 cell growth in a cell viability assay, IC50 = 24.4934 μM. | SANGER | ||
| MPP-89 | Growth Inhibition assay | Inhibition of human MPP-89 cell growth in a cell viability assay, IC50 = 25.6874 μM. | SANGER | ||
| NB10 | Growth Inhibition assay | Inhibition of human NB10 cell growth in a cell viability assay, IC50 = 26.47 μM. | SANGER | ||
| LS-513 | Growth Inhibition assay | Inhibition of human LS-513 cell growth in a cell viability assay, IC50 = 26.8847 μM. | SANGER | ||
| L-540 | Growth Inhibition assay | Inhibition of human L-540 cell growth in a cell viability assay, IC50 = 26.9143 μM. | SANGER | ||
| ES1 | Growth Inhibition assay | Inhibition of human ES1 cell growth in a cell viability assay, IC50 = 27.521 μM. | SANGER | ||
| NTERA-S-cl-D1 | Growth Inhibition assay | Inhibition of human NTERA-S-cl-D1 cell growth in a cell viability assay, IC50 = 30.5093 μM. | SANGER | ||
| EW-1 | Growth Inhibition assay | Inhibition of human EW-1 cell growth in a cell viability assay, IC50 = 32.9454 μM. | SANGER | ||
| Calu-6 | Growth Inhibition assay | Inhibition of human Calu-6 cell growth in a cell viability assay, IC50 = 33.1855 μM. | SANGER | ||
| CTV-1 | Growth Inhibition assay | Inhibition of human CTV-1 cell growth in a cell viability assay, IC50 = 33.9789 μM. | SANGER | ||
| YT | Growth Inhibition assay | Inhibition of human YT cell growth in a cell viability assay, IC50 = 38.5209 μM. | SANGER | ||
| TE-6 | Growth Inhibition assay | Inhibition of human TE-6 cell growth in a cell viability assay, IC50 = 41.2797 μM. | SANGER | ||
| HT-144 | Growth Inhibition assay | Inhibition of human HT-144 cell growth in a cell viability assay, IC50 = 41.5486 μM. | SANGER | ||
| EW-13 | Growth Inhibition assay | Inhibition of human EW-13 cell growth in a cell viability assay, IC50 = 42.2791 μM. | SANGER | ||
| KALS-1 | Growth Inhibition assay | Inhibition of human KALS-1 cell growth in a cell viability assay, IC50 = 43.1328 μM. | SANGER | ||
| MOLT-16 | Growth Inhibition assay | Inhibition of human MOLT-16 cell growth in a cell viability assay, IC50 = 45.0752 μM. | SANGER | ||
| D-336MG | Growth Inhibition assay | Inhibition of human D-336MG cell growth in a cell viability assay, IC50 = 45.9599 μM. | SANGER | ||
| TE-11 | Growth Inhibition assay | Inhibition of human TE-11 cell growth in a cell viability assay, IC50 = 46.653 μM. | SANGER | ||
| EB2 | Growth Inhibition assay | Inhibition of human EB2 cell growth in a cell viability assay, IC50 = 46.699 μM. | SANGER | ||
| SK-N-DZ | Growth Inhibition assay | Inhibition of human SK-N-DZ cell growth in a cell viability assay, IC50 = 48.0961 μM. | SANGER | ||
| SW684 | Growth Inhibition assay | Inhibition of human SW684 cell growth in a cell viability assay, IC50 = 48.2695 μM. | SANGER | ||
| EW-18 | Growth Inhibition assay | Inhibition of human EW-18 cell growth in a cell viability assay, IC50 = 48.4395 μM. | SANGER | ||
| 他の多くの細胞株試験データをご覧になる場合はこちらをクリックして下さい | |||||
生物活性
| 製品説明 | Imatinib Mesylate is an orally bioavailability mesylate salt of Imatinib, which is a multi-target inhibitor of v-Abl, c-Kit and PDGFR with IC50 of 0.6 μM, 0.1 μM and 0.1 μM in cell-free or cell-based assays, respectively. Imatinib Mesylate (STI571) induces autophagy. | ||||||
|---|---|---|---|---|---|---|---|
| Targets |
|
| In Vitro | ||||
| In vitro |
In vitro assays for inhibition of a panel of tyrosine and serine/threonine protein kinases show that Imatinib Mesylate inhibits the v-Abl tyrosine kinase and PDGFR potently with an IC50 of 0.6 and 0.1 μM, respectively. This compound inhibits the SLF-dependent activation of wild-type c-kit kinase activity with a IC50 for these effects of approximately 0.1 μM, which is similar to the concentration required for inhibition of PDGFR. It exhibits growth-inhibitory activity on the human bronchial carcinoid cell line NCI-H727 and the human pancreatic carcinoid cell line BON-1 with an IC50 of 32.4 and 32.8 μM, respectively. A recent study shows that this chemical has the potential to exert its antileukemia effects in chronic myelogenous leukemia by down-regulating hERG1 K(+) channels, which are highly expressed in leukemia cells and appear of exceptional importance in favoring leukemogenesis. |
|||
|---|---|---|---|---|
| Kinase Assay | PDGF receptor kinase activity | |||
| PDGF receptor is immunoprecipitated from BALB/c 3T3 cell extracts with rabbit antiserum to the murine PDGF receptor for 2 hours on ice. Protein A-Sepharose beads are used to collect the antigen-antibody complexes. The immunoprecipitates are washed twice with TNET (50 mM Tris, pH 7.5, 140 mM NaCl, 5 mM EDTA, 1% Triton X-100), once with TNE (50 mM Tris, pH 7.5, 140 mM EDTA), and once with kinase buffer (20 mM Tris, pH 7.5,10 mM MgCl2). After stimulation with PDGF (50 ng/mL) for 10 minutes at 4 °C, different concentrations of this compound are added to the reaction mixture. PDGF receptor kinase activity is determined by incubation with 10 μCi [7-33P]-ATP and l μM ATP for 10 minutes at 4 °C. Immune complexes are separated by SDS-PAGE on 7.5% gels. | ||||
| 細胞実験 | 細胞株 | BON-1 cells and NCI-H727 cells | ||
| 濃度 | ~100 μM | |||
| 反応時間 | 48 hours | |||
| 実験の流れ | BON-1 cells and NCI-H727 cells are seeded into flat-bottomed 96-well plates in triplicate and allowed to adhere overnight in 10% fetal bovine serum-supplemented DMEM or RPMI 1640 complete medium, respectively; the medium is then exchanged for serum-free medium (negative control) or serum-free medium containing serial dilutions of this compound. After 48 hours (control cultures do not reach confluence), the number of metabolically active cells is determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay, and absorbance is measured in a Packard Spectra microplate reader at 540 nm. Growth inhibition is calculated using the following formula: inhibition rate = (1 − a / b) × 100%, where a and b are the absorbance values of the treated and control groups, respectively. |
|||
| 実験結果図 | Methods | Biomarkers | 結果図 | PMID |
| Western blot | p-ZAP70 / ZAP70 / p-LAT / LAT p-STAT3 / STAT3 / p-STAT5 / STAT5 |
|
18981115 | |
| Immunofluorescence | Cortactin / F-actin RelB |
|
20937825 | |
| Growth inhibition assay | Cell viability |
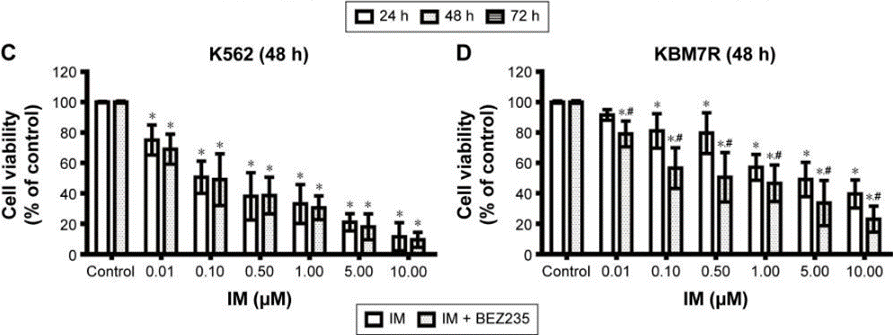
|
28435223 | |
| In Vivo | ||
| In Vivo |
Imatinib Mesylate produces a different antitumor effect on three xenografted tumors derived from surgical samples of fresh human small cell lung cancers, with 80%, 40% and 78% growth inhibition of SCLC6, SCLC61 and SCLC108 tumors, respectively, and no significant inhibition of SCLC74 growth. In high fat fed ApoE(-/-) mice, this compound significantly reduces the high fat-induced lipid staining area by 30%, 27% and 35% compared to high-fat diet untreated controls when dosed by gavage at 10, 20 and 40 mg/kg, respectively, and suppresses carotid artery lipid accumulation. |
|
|---|---|---|
| 動物実験 | 動物モデル | SCLC6, SCLC61, SCLC 74 and SCLC108 small cell lung cancers are injected into Swiss mice (nu/nu, female). |
| 投与量 | 70 or 100 mg/kg | |
| 投与経路 | Administered via i.p. | |
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05623774 | Recruiting | CML |
Inhibikase Therapeutics Inc. |
December 16 2022 | Phase 1 |
| NCT02687425 | Unknown status | Leukemia Myelogenous Chronic BCR-ABL Positive |
Meng Li|Tongji Hospital |
February 2016 | Phase 2 |
| NCT02303899 | Completed | Mesothelioma Malignant |
Istituto Clinico Humanitas |
November 2014 | Phase 2 |
| NCT01898377 | Terminated | Chronic Graft-versus-host Disease |
Seoul National University Hospital |
August 2013 | Phase 2 |
| NCT02891395 | Completed | Graft Versus Host Disease |
University Hospital Lille|Novartis |
December 24 2012 | Phase 2 |
|
化学情報
| 分子量 | 589.71 | 化学式 | C29H31N7O.CH4SO3 |
| CAS No. | 220127-57-1 | SDF | Download Imatinib (STI571) Mesylate SDFをダウンロードする |
| Smiles | CC1=C(C=C(C=C1)NC(=O)C2=CC=C(C=C2)CN3CCN(CC3)C)NC4=NC=CC(=N4)C5=CN=CC=C5.CS(=O)(=O)O | ||
| 保管 | |||
|
In vitro |
DMSO : 118 mg/mL ( (200.09 mM); 吸湿したDMSOは溶解度を減少させます。新しいDMSOをご使用ください。) Water : 118 mg/mL Ethanol : Insoluble |
モル濃度計算器 |
|
in vivo Add solvents to the product individually and in order. |
投与溶液組成計算機 | |||||
実験計算
投与溶液組成計算機(クリア溶液)
ステップ1:実験データを入力してください。(実験操作によるロスを考慮し、動物数を1匹分多くして計算・調製することを推奨します)
mg/kg
g
μL
匹
ステップ2:投与溶媒の組成を入力してください。(ロット毎に適した溶解組成が異なる場合があります。詳細については弊社までお問い合わせください)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
計算結果:
投与溶媒濃度: mg/ml;
DMSOストック溶液調製方法: mg 試薬を μL DMSOに溶解する(濃度 mg/mL, 注:濃度が当該ロットのDMSO溶解度を超える場合はご連絡ください。 )
投与溶媒調製方法:Take μL DMSOストック溶液に μL PEG300,を加え、完全溶解後μL Tween 80,を加えて完全溶解させた後 μL ddH2O,を加え完全に溶解させます。
投与溶媒調製方法:μL DMSOストック溶液に μL Corn oil,を加え、完全溶解。
注意:1.ストック溶液に沈殿、混濁などがないことをご確認ください;
2.順番通りに溶剤を加えてください。次のステップに進む前に溶液に沈殿、混濁などがないことを確認してから加えてください。ボルテックス、ソニケーション、水浴加熱など物理的な方法で溶解を早めることは可能です。
技術サポート
ストックの作り方、阻害剤の保管方法、細胞実験や動物実験の際に注意すべき点など、製品を取扱う時に問い合わせが多かった質問に対しては取扱説明書でお答えしています。
他に質問がある場合は、お気軽にお問い合わせください。
* 必須