- 阻害剤
- 研究分野別
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- 化合物ライブラリー
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-Ⅱ
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at [email protected] to customize your library.
You could select:
- 抗体
- 新製品
- お問い合わせ
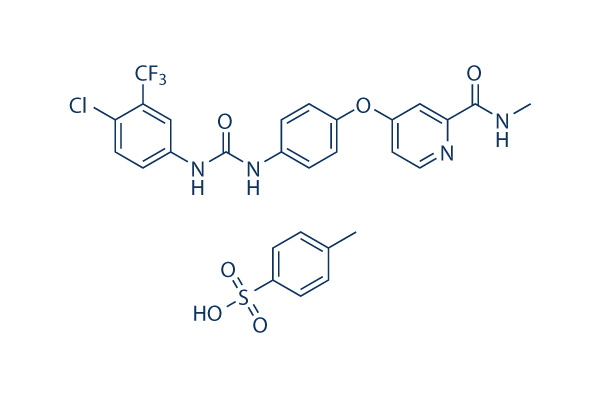
Sorafenib tosylate
別名:BAY 43-9006 tosylate,NSC-724772 tosylate
Sorafenib tosylate is a multikinase inhibitor of Raf-1 and B-Raf with IC50 of 6 nM and 22 nM in cell-free assays, respectively. Sorafenib Tosylate inhibits VEGFR-2, VEGFR-3, PDGFR-β, Flt-3 and c-KIT with IC50 of 90 nM, 20 nM, 57 nM, 59 nM and 68 nM, respectively. Sorafenib Tosylate induces autophagy and apoptosis and activates ferroptosis with anti-tumor activity.
CAS No. 475207-59-1
文献中Selleckの製品使用例(266)
製品安全説明書
現在のバッチを見る:
純度:
99.99%
99.99
Sorafenib tosylate関連製品
シグナル伝達経路
Raf阻害剤の選択性比較
Cell Data
| Cell Lines | Assay Type | Concentration | Incubation Time | 活性情報 | PMID |
|---|---|---|---|---|---|
| HT-29 | Growth Inhibition Assay | 48 h | GI50=2.5 μM | 22560627 | |
| EKVX | Growth Inhibition Assay | 48 h | GI50=2.5 μM | 22560627 | |
| MCF7 | Growth Inhibition Assay | 48 h | GI50=2.5 μM | 22560627 | |
| UACC257 | Growth Inhibition Assay | 48 h | GI50=2 μM | 22560627 | |
| MDA-MB-435 | Growth Inhibition Assay | 48 h | GI50=2 μM | 22560627 | |
| SNB19 | Growth Inhibition Assay | 48 h | GI50=3.2 μM | 22560627 | |
| OVCAR3 | Growth Inhibition Assay | 48 h | GI50=3.2 μM | 22560627 | |
| CAKI-1 | Growth Inhibition Assay | 48 h | GI50=3.2 μM | 22560627 | |
| SW620 | Growth Inhibition Assay | 48 h | GI50=3.2 μM | 22560627 | |
| TK10 | Growth Inhibition Assay | 48 h | GI50=5 μM | 22560627 | |
| endothelial precursor cells | Function assay | Inhibition of endothelial cord area formation in endothelial precursor cells by CD31 cord area detection based phenotypic drug discovery based assay, IC50 = 0.00421 μM. | 22409666 | ||
| Sf9 | Function assay | Inhibition of GST-tagged recombinant human VEGFR2 expressed in Sf9 cells by radiometric assay, IC50 = 0.0125 μM. | 24368209 | ||
| endothelial precursor cells/ADSC cells | Toxicity assay | Toxicity in endothelial precursor cells co-cultured with stromal precursor ADSC cells by total nuclei count detection based phenotypic drug discovery based assay, EC50 = 5.46 μM. | 22409666 | ||
| endothelial precursor cells | Function assay | Inhibition of cell migration in endothelial precursor cells by Oris cell migration kit based phenotypic drug discovery based assay, IC50 = 16.7 μM. | 22409666 | ||
| RD | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for RD cells | 29435139 | ||
| SK-N-SH | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-SH cells | 29435139 | ||
| OHS-50 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for OHS-50 cells | 29435139 | ||
| 他の多くの細胞株試験データをご覧になる場合はこちらをクリックして下さい | |||||
生物活性
| 製品説明 | Sorafenib tosylate is a multikinase inhibitor of Raf-1 and B-Raf with IC50 of 6 nM and 22 nM in cell-free assays, respectively. Sorafenib Tosylate inhibits VEGFR-2, VEGFR-3, PDGFR-β, Flt-3 and c-KIT with IC50 of 90 nM, 20 nM, 57 nM, 59 nM and 68 nM, respectively. Sorafenib Tosylate induces autophagy and apoptosis and activates ferroptosis with anti-tumor activity. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Targets |
|
| In Vitro | ||||
| In vitro |
Sorafenib tosylate inhibits both wild-type and V599E mutant B-Raf activity with IC50 of 22 nM and 38 nM, respectively. Sorafenib tosylate also potently inhibits mVEGFR2 (Flk-1), mVEGFR3, mPDGFRβ, Flt3, and c-Kit with IC50 of 15 nM, 20 nM, 57 nM, 58 nM, and 68 nM, respectively. Sorafenib tosylate weakly inhibits FGFR-1 with IC50 of 580 nM. Sorafenib tosylate is not active against ERK-1, MEK-1, EGFR, HER-2, IGFR-1, c-Met, PKB, PKA, cdk1/cyclinB, PKCα, PKCγ, and pim-1. Sorafenib tosylate markedly inhibits VEGFR2 phosphorylation in NIH 3T3 cells with IC50 of 30 nM, and Flt-3 phosphorylation in HEK-293 cells with IC50 of 20 nM. Sorafenib tosylate potently blocks MEK 1/2 and ERK 1/2 phosphorylation in most cell lines but not in A549 or H460 cells, while having no effect on inhibition of the PKB pathway. Sorafenib tosylate inhibits the proliferation of HAoSMC and MDA-MB-231 cells with IC50 of 0.28 μM and 2.6 μM, respectively. [1] In addition to inhibition of the RAF/MEK/ERK signaling pathway, Sorafenib tosylate significantly inhibits the phosphorylation of eIF4E and down-regulates Mcl-1 levels in hepatocellular carcinoma (HCC) cells in a MEK/ERK-independent manner. Sorafenib tosylate inhibits the proliferation of PLC/PRF/5 and HepG2 cells with IC50 of 6.3 μM and 4.5 μM, respectively, and leads to the significant induction of apoptosis. [2] |
|||
|---|---|---|---|---|
| Kinase Assay | Biochemical assays | |||
| Recombinant baculoviruses expressing Raf-1 (residues 305–648) and B-Raf (residues 409–765) are purified as fusion proteins. Full-length human MEK-1 is generated by PCR and purified as a fusion protein from Escherichia coli lysates. Sorafenib tosylate is added to a mixture of Raf-1 (80 ng), or B-Raf (80 ng) with MEK-1 (1 μg) in assay buffer [20 mM Tris (pH 8.2), 100 mM NaCl, 5 mM MgCl2, and 0.15% β-mercaptoethanol] at a final concentration of 1% DMSO. The Raf kinase assay (final volume of 50 μL) is initiated by adding 25 μL of 10 μM γ[33P]ATP (400 Ci/mol) and incubated at 32 °C for 25 minutes. Phosphorylated MEK-1 is harvested by filtration onto a phosphocellulose mat, and 1% phosphoric acid is used to wash away unbound radioactivity. After drying by microwave heating, a β-plate counter is used to quantify filter-bound radioactivity. Human VEGFR2 (KDR) kinase domain is expressed and purified from Sf9 lysates. Time-resolved fluorescence energy transfer assays for VEGFR2 are performed in 96-well opaque plates in the time-resolved fluorescence energy transfer format. Final reaction conditions are as follows: 1 to 10 μM ATP, 25 nM poly GT-biotin, 2 nM Europium-labeled phospho (p)-Tyr antibody (PY20), 10 nM APC, 1 to 7 nM cytoplasmic kinase domain in final concentrations of 1% DMSO, 50 mM HEPES (pH 7.5), 10 mM MgCl2, 0.1 mM EDTA, 0.015% Brij-35, 0.1 mg/mL BSA, and 0.1% β-mercaptoethanol. Reaction volumes are 100 μL and are initiated by addition of enzyme. Plates are read at both 615 and 665 nM on a Perkin-Elmer VictorV Multilabel counter at ~1.5 to 2.0 hours after reaction initiation. Signal is calculated as a ratio: (665 nm/615 nM) × 10,000 for each well. For IC50 generation, Sorafenib tosylate is added before the enzyme initiation. A 50-fold stock plate is made with Sorafenib tosylate serially diluted 1:3 in a 50% DMSO/50% distilled water solution. Final Sorafenib tosylate concentrations range from 10 μM to 4.56 nM in 1% DMSO. | ||||
| 細胞実験 | 細胞株 | MDA-MB-231, and HAoSMC | ||
| 濃度 | Dissolved in DMSO, final concentrations ~10 μM | |||
| 反応時間 | 72 hours | |||
| 実験の流れ | Cells are exposed to increasing concentrations of Sorafenib tosylate for 72 hours. Cell number is quantitated using the Cell TiterGlo ATP Luminescent assay kit. This assay measures the number of viable cells per well by measurement of luminescent signal based on amount of cellular ATP. |
|||
| 実験結果図 | Methods | Biomarkers | 結果図 | PMID |
| Western blot | LC3-I / LC-3II / ATG5 p-STAT3 / STAT3/ Mcl-1 β-catenin / Survivin / Mcl-1 / PTMA pERK / ERK p-PKM2(Y105) / PMK2 / Caspase-9 RET(pY1016) / VEGFR2(pY1214) / MEK1(pT292) / ERK(pY204) Cyclin D1 |
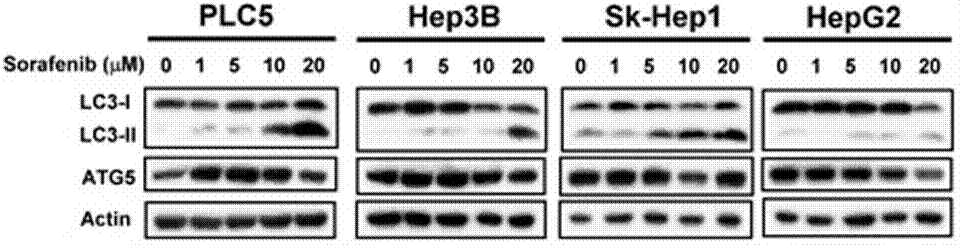
|
23392173 | |
| Immunofluorescence | p65 cytochrome c |
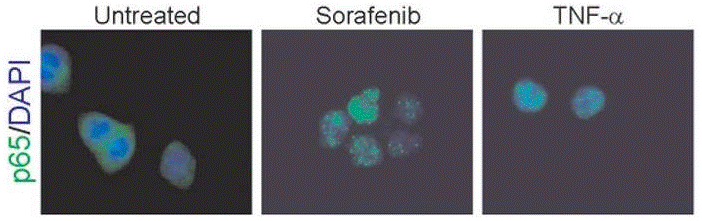
|
22286758 | |
| Growth inhibition assay | Cell viability |
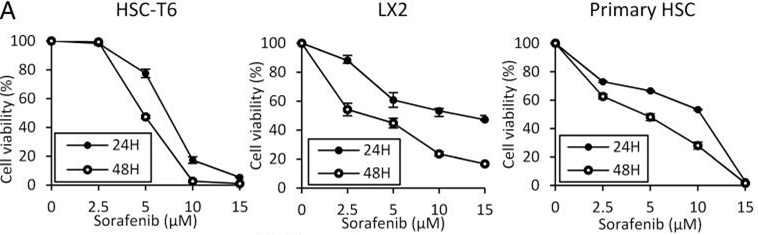
|
26039995 | |
| ELISA | TGF-beta / CD206 Caspase-9 / Caspase-3 |
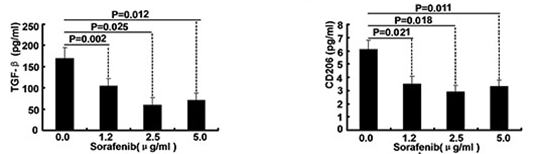
|
26158762 | |
| In Vivo | ||
| In Vivo |
Oral administration of Sorafenib tosylate (~60 mg/kg) demonstrates broad spectrum, dose-dependent anti-tumor activity against a variety of human tumor xenograft models including MDA-MB-231, Colo-205, HT-29, DLD-1, NCI-H460, and A549, with no evidence of toxicity. In association with the anti-tumor efficacy, Sorafenib tosylatetreatment potently inhibits MEK 1/2 phosphorylation and pERK 1/2 levels in HT-29 and MDA-MB-231 xenografts but not in Colo-205 xenografts, and significantly suppresses tumor microvessel area (MVA) and microvessel density (MVD) in MDA MB-231, HT-29 and Colo-205 tumor xenografts. [1] Sorafenib tosylate treatment produces dose-dependent growth inhibition of PLC/PRF/5 tumor xenografts in SCID mice with TGIs of 49% and 78% at 10 mg/kg and 30 mg/kg, respectively, consistent with the inhibition of ERK and eIF4E phosphorylation, reduction of the microvessel area, and induction of tumor cell apoptosis. [2] |
|
|---|---|---|
| 動物実験 | 動物モデル | Female NCr-nu/nu mice implanted s.c. with MDA-MB-231, Colo-205, HT-29, H460, or A549 cells |
| 投与量 | ~60 mg/kg | |
| 投与経路 | Orally once daily | |
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05068752 | Recruiting | Pancreas Cancer |
HonorHealth Research Institute|Bayer|Genentech Inc. |
October 28 2021 | Phase 2 |
| NCT04763408 | Active not recruiting | Carcinoma Hepatocellular |
Eisai Inc. |
April 9 2021 | -- |
化学情報
| 分子量 | 637.03 | 化学式 | C21H16ClF3N4O3.C7H8O3S |
| CAS No. | 475207-59-1 | SDF | Download Sorafenib tosylate SDFをダウンロードする |
| Smiles | CC1=CC=C(C=C1)S(=O)(=O)O.CNC(=O)C1=NC=CC(=C1)OC2=CC=C(C=C2)NC(=O)NC3=CC(=C(C=C3)Cl)C(F)(F)F | ||
| 保管 | |||
|
In vitro |
DMSO : 127 mg/mL ( (199.36 mM); 吸湿したDMSOは溶解度を減少させます。新しいDMSOをご使用ください。) Water : 0.01 mg/mL Ethanol : Insoluble |
モル濃度計算器 |
|
in vivo Add solvents to the product individually and in order. |
投与溶液組成計算機 | ||||
実験計算
投与溶液組成計算機(クリア溶液)
ステップ1:実験データを入力してください。(実験操作によるロスを考慮し、動物数を1匹分多くして計算・調製することを推奨します)
mg/kg
g
μL
匹
ステップ2:投与溶媒の組成を入力してください。(ロット毎に適した溶解組成が異なる場合があります。詳細については弊社までお問い合わせください)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
計算結果:
投与溶媒濃度: mg/ml;
DMSOストック溶液調製方法: mg 試薬を μL DMSOに溶解する(濃度 mg/mL, 注:濃度が当該ロットのDMSO溶解度を超える場合はご連絡ください。 )
投与溶媒調製方法:Take μL DMSOストック溶液に μL PEG300,を加え、完全溶解後μL Tween 80,を加えて完全溶解させた後 μL ddH2O,を加え完全に溶解させます。
投与溶媒調製方法:μL DMSOストック溶液に μL Corn oil,を加え、完全溶解。
注意:1.ストック溶液に沈殿、混濁などがないことをご確認ください;
2.順番通りに溶剤を加えてください。次のステップに進む前に溶液に沈殿、混濁などがないことを確認してから加えてください。ボルテックス、ソニケーション、水浴加熱など物理的な方法で溶解を早めることは可能です。
技術サポート
ストックの作り方、阻害剤の保管方法、細胞実験や動物実験の際に注意すべき点など、製品を取扱う時に問い合わせが多かった質問に対しては取扱説明書でお答えしています。
他に質問がある場合は、お気軽にお問い合わせください。
* 必須
Tags: Sorafenib tosylateを買う | Sorafenib tosylate ic50 | Sorafenib tosylate供給者 | Sorafenib tosylateを購入する | Sorafenib tosylate費用 | Sorafenib tosylate生産者 | オーダーSorafenib tosylate | Sorafenib tosylate化学構造 | Sorafenib tosylate分子量 | Sorafenib tosylate代理店