
- 阻害剤
- 研究分野別
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- 化合物ライブラリー
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-II
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at info@selleck.co.jp to customize your library.
You could select:
- 抗体
- 新製品
- お問い合わせ
invivo抗体
| 製品コード | 製品名 | Application | Product Validations | 製品説明 |
|---|---|---|---|---|
| A2115 | Anti-mouse PD-L1 (B7-H1)-InVivo | in vivo PD-L1 blockade,IHC (frozen),FCM |
-InVivo.gif)
|
Anti-mouse PD-L1 (B7-H1)-InVivo (Clone:10F.9G2) monoclonal antibody reacts with mouse PD-L1 (also known as B7-H1, CD274). The 10F.9G2 antibody has been shown to block the interaction between PD-L1 and PD-1 and between PD-L1 and B7-1 (CD80). |
| A2122 | Anti-mouse PD-1 (CD279)-InVivo |
-InVivo.gif)
|
Anti-mouse PD-1 (CD279)-InVivo (Clone:RMP1-14)reacts with mouse PD-1 (programmed death-1) also known as CD279. PD-1 is a 50-55 kDa cell surface receptor encoded by the Pdcd1 gene that belongs to the CD28 family of the Ig superfamily. | |
| A2102 | Anti-mouse CD8α-InVivo | In vivo CD8+ T cell depletion,WB |

|
Anti-mouse CD8α (Clone:2.43)reacts with mouse CD8α. CD8 is a transmembrane glycoprotein that acts as a co-receptor for the T cell receptor (TCR). It binds to class I MHC molecules displayed by antigen presenting cells (APC). |
| A2111 | Anti-human CD3-InVivo | in vitro T cell stimulation/activation, FCM | The OKT-3 monoclonal antibody reacts with human CD3ε which is a 20 kDa transmembrane cell-surface protein that belongs to the immunoglobulin superfamily.The OKT-3 antibody has immunosuppressive properties in vivo and has been shown to effectively treat renal, heart and liver allograft rejection. | |
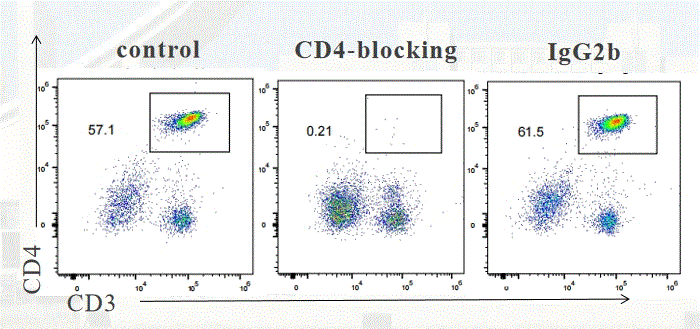
| A2101 | Anti-mouse CD4-InVivo | In vivo CD4+ T cell depletion,FCM,WB |

|
Anti-mouse CD4 antibody(Clone:GK1.5) reacts with mouse CD4, which is a 55 kDa cell surface type I membrane glycoprotein. CD4 is a co-receptor of the T cell receptor (TCR) and assists the latter in communicating with antigen-presenting cells. |
| A2116 | Rat IgG2b isotype control-InVivo |
|
Rat IgG2b isotype control-InVivo monoclonal antibody (Clone:LTF-2) reacts with keyhole limpet hemocyanin (KLH). Because KLH is not expressed by mammals this antibody is ideal for use as an isotype-matched control for rat IgG2b antibodies in most in vivo and in vitro applications. | |
| A2110 | Anti-mouse CD16/CD32-InVivo | in vitro/vivo Fc receptor blocking |

|
The 2.4G2 monoclonal antibody(Clone: 2.4G2) reacts specifically with mouse CD16 (FcγRIII) and CD32 (FcγRII). It has also been reported to react non-specifically via its Fc domain to FcγRI. The 2.4G2 antibody is commonly used in flow cytometry staining experiments to prevent non-specific binding of IgG to the FcγIII and FcγII, and possibly FcγI, receptors prior to staining with antigen specific primary antibodies. |
| A2103 | Anti-mouse CTLA-4 (CD152)-InVivo | in vivo/vitro CTLA-4 neutralization, FCM,WB | Anti-mouse CTLA-4 (CD152) antibody (Clone:9H10) reacts with mouse CTLA-4 (cytotoxic T lymphocyte antigen-4) also known as CD152. Anti-mouse CTLA-4 (CD152) antibody has been shown to promote T cell co-stimulation by blocking CTLA-4 binding to the B7 co-receptors, allowing for CD28 binding. | |
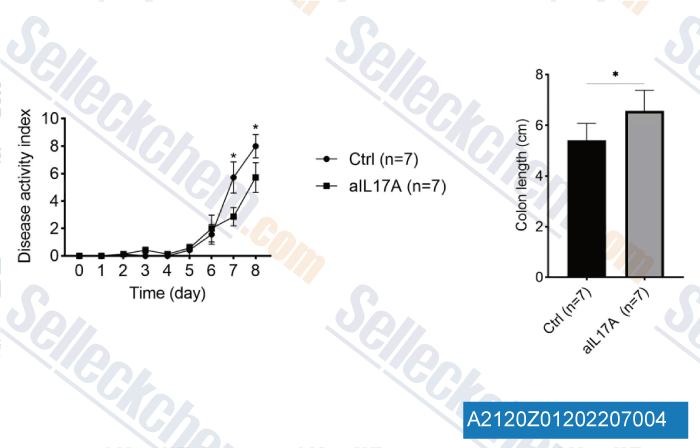
| A2120 | Anti-mouse IL-17A-InVivo | in vivo IL-17A neutralization |
|
Anti-mouse IL-17A-InVivo monoclonal antibody (Clone:17F3) reacts with mouse IL-17A which is a 15-20 kDa cytokine expressed by Th17 cells, γδ T cells, iNKT cells, NK cells, LTi cells, neutrophils, and intestinal Paneth cells. Anti-mouse IL-17A-InVivo has been shown to neutralize IL-17A in vivo. |
| A2113 | Anti-human/mouse TGF-β-InVivo | in vivo/vitro TGFβ neutralization,WB | The 1D11.16.8 monoclonal antibody reacts with mouse, human, rat, monkey, hamster, canine and bovine TGF-β (transforming growth factor beta) isoforms 1, 2 and 3.The 1D11.16.8 monoclonal antibody is a neutralizing antibody. | |
| A2121 | Anti-mouse IFNAR-1-InVivo | in vivo IFNAR-1 blockade, in vitro IFNAR-1 blockade, WB |

|
Anti-mouse IFNAR-1-InVivo monoclonal antibody (Clone: MAR1-5A3) reacts with mouse IFNAR-1 (IFN alpha/beta receptor subunit 1). Anti-mouse IFNAR-1-InVivo has been shown to inhibit Type I IFN receptor signaling in vitro and in vivo. |
| A2117 | Mouse IgG2a isotype control-InVivo | Mouse IgG2a isotype control-InVivo (Clone:C1.18.4) monoclonal antibody is ideal for use as a non-reactive isotype-matched control for mouse IgG2a antibodies in most in vivo and in vitro applications. | ||
| A2104 | Anti-mouse CD3ε-InVivo | in vitro T cell stimulation/activation, IF, FCM, WB | The 145-2C11 monoclonal antibody reacts with mouse CD3ε which is a 20 kDa transmembrane cell-surface protein that belongs to the immunoglobulin superfamily. The 145-2C11 antibody has been shown to induce T lymphocyte activation, proliferation, and apoptosis in vitro via binding and stimulating the TCR. When used in vivo the antibody is reported to produce T cell activation, anergy or death. | |
| A2118 | Anti-mouse IL-6-InVivo | in vivo IL-6 neutralization |

|
Anti-mouse IL-6-InVivo monoclonal antibody (Clone:MP5-20F3) can reacts with mouse IL-6 (interleukin-6) which is a 21-28 kDa cytokine that is expressed by many cell types, including T lymphocytes, B lymphocytes, monocytes, fibroblasts, and endothelial cells. Anti-mouse IL-6-InVivo has been shown to neutralize the bioactivity of natural or recombinant IL-6. |
| A2108New | Anti-mouse CD28-InVivo | in vitro T cell stimulation/activation | The PV-1 monoclonal antibody(Clone: PV-1) reacts with mouse CD28.The PV-1 antibody has been shown to stimulate the proliferation and cytokine production by activated T and NK cells. | |
| A2119 | Rat IgG1 isotype control-InVivo |

|
Rat IgG1 isotype control-InVivo monoclonal antibody (Clone:HRPN) reacts with horseradish peroxidase (HRP). Because HRP is not expressed by mammals this antibody is ideal for use as an isotype-matched control for rat IgG1 antibodies in most in vivo and in vitro applications. | |
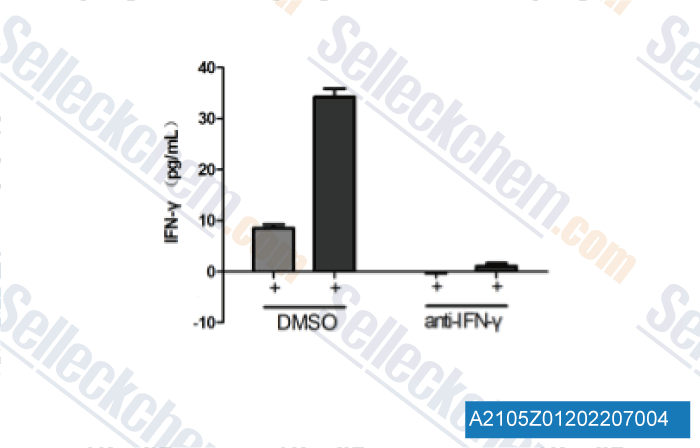
| A2105 | Anti-mouse IFNγ-InVivo | in vivo/vitro IFNγ neutralization |
|
The R4-6A2 monoclonal antibody reacts with mouse IFNγ (interferon gamma) which is a 20 kDa soluble pleiotropic cytokine and the sole member of the type II class of interferons. The R4-6A2 antibody has been shown to neutralize both natural and recombinant IFNγ. |
| A2109 | Anti-mouse IL-4-InVivo | in vivo/vitro IL-4 neutralization,FCM,WB | The 11B11 monoclonal antibody reacts with mouse IL-4 (interleukin-4) which is a multifunctional 14 kDa cytokine.The 11B11 monoclonal antibody has been shown to neutralize the bioactivity of natural or recombinant IL-4. | |
| A2149 | Anti-mouse CD19-InVivo | Anti-mouse CD19-InVivo |

|
Anti-mouse CD19-InVivo reacts with mouse CD19, a B cell-specific 95 kDa transmembrane glycoprotein of the immunoglobulin superfamily. |
| A2159 | Anti-mouse CSF1R (CD115)-InVivo |

|
Anti-mouse CSF1R (CD115)-InVivo reacts with mouse colony stimulating factor 1 receptor (CSF1R), also known as macrophage colony-stimulating factor receptor (M-CSFR), and CD115. CSF1R is a single-pass type I membrane protein and member of the platelet-derived growth factor receptor family. | |
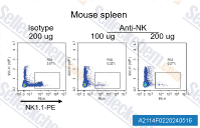
| A2114 | Anti-mouse NK1.1-InVivo | in vivo NK cell depletion,FCM |
|
The PK136 monoclonal antibody(Clone: PK136) reacts with mouse NK1.1 also known as CD161b/CD161c, KLRB1, NKR-P1A and Ly-55. |
| A2107 | Anti-mouse CD25 (IL-2Rα)-InVivo | in vivo regulatory T cell depletion,FCM |
-InVivo.gif)
|
The PC-61.5.3 monoclonal antibody reacts with mouse IL-2Rα also known as CD25, Ly-43, p55, or Tac.The PC-61.5.3 antibody has been shown to inhibit the binding of IL-2 to both the low and high affinity IL-2 receptor forms. Additionally, the PC-61.5.3 antibody is commonly used to deplete CD4+FoxP3+ T regulatory cells in vivo. |
| A2141 | Anti-mouse Ter-119-InVivo | Anti-mouse Ter-119-InVivo | Anti-mouse Ter-119-InVivo reacts with mouse Ter-119, a 52 kDa glycophorin A-associated protein that is expressed by erythroid cells from the early proerythroblast stage to mature erythrocytes. Anti-mouse Ter-119-InVivo is commonly used for identifying erythrocytes and cells in the erythroid lineage. | |
| A2168 | Anti-human CD3 (UCHT1)-InVivo | Anti-human CD3 (UCHT1)-InVivo reacts with human CD3ε a 20 kDa transmembrane cell-surface protein that belongs to the immunoglobulin superfamily. | ||
| A2142 | Anti-mouse/rat IL-1β-InVivo | Anti-mouse/rat IL-1β-InVivo | Anti-mouse/rat IL-1β-InVivo reacts with precursor and mature secreted forms of mouse and rat IL-1β a 17 kDa pro-inflammatory cytokine produced primarily by monocytes. | |
| A2143 | Anti-mouse 4-1BB (CD137)-InVivo | Anti-mouse 4-1BB (CD137)-InVivo | Anti-mouse 4-1BB (CD137)-InVivo reacts with mouse 4-1BB, a TNF receptor superfamily member also known as CD137. | |
| A2144 | Anti-mouse TIGIT-InVivo | Anti-mouse TIGIT-InVivo | Anti-mouse TIGIT-InVivo reacts with mouse TIGIT (T cell immunoreceptor with Ig and ITIM domains). TIGIT is a 26 kDa, type I transmembrane protein and a member of the poliovirus receptor (PVR) family. | |
| A2145 | Mouse IgG2b isotype control-InVivo | Mouse IgG2b isotype control-InVivo | Mouse IgG2b isotype control-InVivo is ideal for use as a non-reactive isotype-matched control for mouse IgG2b antibodies in most in vivo and in vitro applications. | |
| A2165 | Anti-mouse PD-1 (CD279)(29F.1A12)-InVivo | in vivo blocking of PD-1/PD-L signaling in vitro PD-1 neutralization | ||
| A2146 | Anti-LCMV nucleoprotein-InVivo | Anti-LCMV nucleoprotein-InVivo | Anti-LCMV nucleoprotein-InVivo reacts with lymphocytic choriomeningitis virus (LCMV) nucleoprotein (NP), a 63 kDa structural protein. | |
| A2147 | Anti-mouse CXCR3 (CD183)-InVivo | Anti-mouse CXCR3 (CD183)-InVivo | Anti-mouse CXCR3 (CD183)-InVivo reacts with mouse CXCR3 also known as CD183, a 38 kDa chemokine receptor for CXCL9 (MIG), CXCL10 (IP-10), and CXCL11 (ITAC). | |
| A2148 | Anti-mouse GM-CSF-InVivo | Anti-mouse GM-CSF-InVivo | Anti-mouse GM-CSF-InVivo reacts with mouse granulocyte-macrophage colony-stimulating factor (GM-CSF), also known as colony stimulating factor 2 (CSF2). | |
| A2150 | Armenian hamster IgG isotype control-InVivo | Armenian hamster IgG-InVivo | ||
| A2151 | Anti-mouse IL-10R (CD210)-InVivo | Anti-mouse IL-10R (CD210)-InVivo |

|
Anti-mouse IL-10R (CD210)-InVivo reacts with mouse IL-10R (IL-10 receptor) also known as CD210. The IL-10R is a class II cytokine receptor and is expressed by a variety of cell types including thymocytes, T lymphocytes, B lymphocytes, NK cells, monocytes, and macrophages. |
| A2152 | Anti-mouse IFNγ (XMG1.2)-InVivo | Anti-mouse IFNγ (XMG1.2)-InVivo | Anti-mouse IFNγ (XMG1.2)-InVivo reacts with mouse IFNγ (interferon gamma) a 20 kDa soluble pleiotropic cytokine and the sole member of the type II class of interferons. | |
| A2153 | Anti-mouse IL-2 (S4B6-1)-InVivo | Anti-mouse IL-2 (S4B6-1)-InVivo | Anti-mouse IL-2 (S4B6-1)-InVivo reacts with mouse IL-2, a 17 kDa cytokine that is mainly produced by T cells in response to antigenic or mitogenic stimulation. | |
| A2154 | Anti-mouse CD86 (B7-2)-InVivo | Anti-mouse CD86 (B7-2)-InVivo | Anti-mouse CD86 (B7-2)-InVivo reacts with mouse CD86 also known as B7-2. CD86 is an 80 kDa Ig superfamily member. | |
| A2156 | Anti-human PD-L1 (B7-H1)-InVivo | Anti-human PD-L1 (B7-H1)-InVivo | Anti-human PD-L1 (B7-H1)-InVivo reacts with human PD-L1 (programmed death ligand 1) also known as B7-H1 or CD274. PD-L1 is a 40 kDa type I transmembrane protein that belongs to the B7 family of the Ig superfamily. | |
| A2157 | Anti-mouse IL-6R-InVivo | Anti-mouse IL-6R-InVivo | Anti-mouse IL-6R-InVivo reacts with the mouse IL-6 receptor α chain also known as CD126. CD126 is an 80 kDa type I cytokine receptor and a member of the immunoglobulin superfamily. | |
| A2158 | Anti-mouse Ly6G-InVivo | Anti-mouse Ly6G-InVivo |
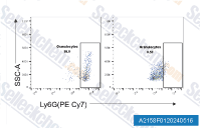
|
Anti-mouse Ly6G-InVivo reacts with mouse Ly6G. Ly6G is a 21-25 kDa member of the Ly-6 superfamily of GPI-anchored cell surface proteins with roles in cell signaling and cell adhesion. |
| A2126 | Anti-mouse VEGFR-2-InVivo | Anti-mouse VEGFR-2-InVivo | Anti-mouse VEGFR-2-InVivo (Clone: DC101) monoclonal antibody reacts with mouse VEGFR-2 (vascular endothelial growth factor receptor 2) also known as CD309, KDR, and Flk-1 and inhibit VEGFR-2 signaling in vivo. VEGFR-2 plays a key role in vascular development and permeability. | |
| A2127 | Anti-human CD28-InVivo | Anti-human CD28-InVivo | Anti-human CD28-InVivo (Clone: 9.3) monoclonal antibody reacts with human CD28, a 45 kDa costimulatory receptor and a member of the Ig superfamily. It stimulate the proliferation of human T cells in vitro. | |
| A2129 | Anti-mouse Thy1.2 (CD90.2)-InVivo | Anti-mouse Thy1.2 (CD90.2)-InVivo | Anti-mouse Thy1.2 (CD90.2)-InVivo(clone 30H12) is a monoclonal antibody reacts with mouse Thy1.2 also known as CD90.2. It induces Ca2+ flux in thymocytes and is particularly useful for depleting T lymphocytes. | |
| A2130 | Anti-mouse LFA-1α (CD11a)-InVivo | Anti-mouse LFA-1α (CD11a)-InVivo | Anti-mouse LFA-1α (CD11a)-InVivo(clone M17/4) is a monoclonal antibody reacts with mouse LFA-1α (lymphocyte function-associated antigen 1 alpha) also known as integrin alpha L chain and CD11a. It plays a central role in leukocyte intercellular adhesion through interactions with its ligands, ICAM-1 (CD54), ICAM-2 (CD102), and ICAM-3 (CD50), and also functions in lymphocyte costimulatory signaling. | |
| A2131 | Anti-mouse TIM-3 (CD366)-InVivo | Anti-mouse TIM-3 (CD366)-InVivo | Anti-mouse TIM-3 (CD366)-InVivo monoclonal antibody reacts with mouse TIM-3 (T cell immunoglobulin and mucin domain-3) also known as CD366. TIM-3 is specifically expressed at high levels on the surface of Th1 lymphocytes. This B8.2C12 antibody binds to the BALB/c allele of TIM-3 while reactivity to the C57Bl/6 allele. Inhibition of TIM-3 signaling in mice exacerbates experimental autoimmune encephalomyelitis and promotes IFNγ production and Th1 cell proliferation. | |
| A2132 | Anti-mouse/human/rat CCL2 (MCP-1)-InVivo | in vivo CCL2 neutralization, Immunohistochemistry (frozen) | Anti-mouse/human/rat CCL2 (MCP-1)-InVivo (Clone: 2H5) antibody reacts with mouse, human, and rat CCL2, also known as monocyte chemoattractant protein 1 (MCP-1). MCP-1 is a 13 kDa chemokine produced mainly by monocytes/macrophages and is a potent monocyte-attracting chemokine. The 2H5 antibody has been shown to neutralize the biological effects of CCL2 in vivo. | |
| A2133 | Anti-mouse LAG-3-InVivo | in vitro &in vivo LAG-3 neutralization, Flow cytometry, Western blot |

|
Anti-mouse LAG-3-InVivo (Clone: C9B7W) monoclonal antibody reacts with mouse LAG-3 also known as CD223. LAG-3 promotes immune responses by activating antigen-presenting cells. The C9B7W antibody has been reported to block the function of murine LAG-3 in vivo and in vitro. |
| A2134 | Anti-mouse IL-18-InVivo | in vitro &in vivo IL-18 neutralization | Anti-mouse IL-18-InVivo (Clone:YIGIF74-1G7) monoclonal antibody reacts with mouse IL-18. IL-18 an 18 kDa pro-inflammatory cytokine is expressed by activated macrophages, keratinocytes, Kupffer cells, intestinal epithelial cells, and osteoblasts. | |
| A2135New | Anti-mouse Ly6G/Ly6C (Gr-1)-InVivo | in vivo depletion of Gr-1+ myeloid cells, Flow cytometry, Immunohistochemistry (paraffin), Immunohistochemistry (frozen) | Anti-mouse Ly6G/Ly6C (Gr-1)-InVivo reacts strongly with mouse Ly6G and weakly with mouse Ly6C previously referred to as GR-1. Ly6G is a 21-25 kDa member of the Ly-6 superfamily of GPI-anchored cell surface proteins with roles in cell signaling and cell adhesion. | |
| A2173New | Anti-mouse L-Selectin (CD62L)-InVivo | in vivo CD62L neutralization. | ||
| A2174New | Anti-mouse/human CD44-InVivo | in vivo/vitro CD44 neutralization. | ||
| A2160 | Anti-rat Kappa Immunoglobulin Light Chain-InVivo | Anti-rat Kappa Immunoglobulin Light Chain-InVivo | Anti-rat Kappa Immunoglobulin Light Chain-InVivo reacts with the kappa chain of the rat immunoglobulin light chain. The κ chain is one of two types of polypeptide subunits which make up the immunoglobulin light chain. | |
| A2161 | Anti-mouse ICOSL (CD275)-InVivo | Anti-mouse ICOSL (CD275)-InVivo | Anti-mouse ICOSL (CD275)-InVivo reacts with mouse ICOSL (inducible T cell co-stimulator ligand) also known as CD275, B7RP-1, and B7-H2. ICOSL is a 40 kDa immune checkpoint protein belonging to the Ig receptor superfamily. | |
| A2162 | Anti-mouse/human VLA-4 (CD49d)-InVivo | Anti-mouse/human VLA-4 (CD49d)-InVivo | ||
| A2166New | Anti-mouse CD28(37.51)-InVivo | Anti-mouse CD28(37.51)-InVivo | Anti-mouse CD28(37.51)-InVivo reacts with mouse CD28, a 45 kDa costimulatory receptor and a member of the Ig superfamily. CD28 is expressed by thymocytes, most peripheral T cells, and NK cells. | |
| A2172New | Anti-human MHC Class I (HLA-A, HLA-B, HLA-C)-InVivo | Anti-human MHC Class I (HLA-A, HLA-B, HLA-C)-InVivo | ||
| A2169 | Anti-mouse MHC Class II (I-A/I-E)-InVivo | Anti-mouse MHC Class II (I-A/I-E)-InVivo | ||
| A2170 | Anti-mouse CSF1-InVivo | Anti-mouse CSF1-InVivo | ||
| A2106 | Mouse IgG1 isotype control-InVivo | FCM,IHC,IP,WB | The monoclonal mouse IgG1 K immunoglobulin is ideal for use as a non-reactive isotype-matched control for mouse IgG1 antibodies in most in vivo and in vitro applications. | |
| A2123 | Rat IgG2a isotype control-InVivo | Rat IgG2a isotype control-InVivo can be used as an isotype-matched control for rat IgG2a antibodies. | ||
| A2112 | Anti-mouse CD40L (CD154)-InVivo | in vivo/vitro blocking of CD40/CD40L,WB | The MR-1 monoclonal antibody reacts with mouse CD154 also known as CD40 ligand. The MR-1 monoclonal antibody has been reported to inhibit in vitro activation of B lymphocytes by blocking the binding of CD154 with CD40 on T helper cells as well as inhibit the formation of germinal centers and disrupt antigen-specific T cell responses. Additionally, the MR-1 antibody blocks interactions of T cells and antigen-presenting cells in vitro and blocks the development of experimental autoimmune disease in vivo. | |
| A2167 | Anti-mouse CD3-InVivo | Anti-mouse CD3-InVivo | Anti-mouse CD3-InVivo reacts with mouse CD3, a transmembrane cell-surface protein that belongs to the immunoglobulin superfamily.CD3 is expressed on T lymphocytes, NK-T cells, and to varying degrees on developing thymocytes. | |
| A2163 | Anti-mouse IL-12 p40-InVivo | Anti-mouse IL-12 p40-InVivo | ||
| A2164 | Anti-mouse B220-InVivo | Anti-mouse B220-InVivo | ||
| A2124 | Anti-mouse TNFα-InVivo | in vitro &in vivo TNFα neutralization, Western blot | Anti-mouse TNFα-InVivo reacts with mouse TNFα (tumor necrosis factor-alpha), a multifunctional proinflammatory cytokine. TNFα dysregulation is been implicated in a variety of diseases, including autoimmune diseases, insulin resistance, and cancer. TNFα knockout animals display defects in response to bacterial infection, characterized by defects in forming organized follicular dendritic cell networks and germinal centers with a lack of primary B cell follicles. | |
| A2125 | Anti-mouse OX40 (CD134)-InVivo | in vitro &in vivo OX40 activation, Western blot | Anti-mouse OX40 (CD134)-InVivo (Clone: OX-86) monoclonal antibody reacts with mouse OX-40 also known as CD134. OX-40 plays a major role in regulating both CD4 and CD8 T cell clonal expansion. Anti-mouse OX40 (CD134)-InVivo treatment strongly enhances the generation of antigen-specific effector T cells, prevents the induction of T cell tolerance, and delays tumor growth in vivo. | |
| A2171New | Anti-mouse PD-L1 (B7-H1) (10F.2H11)-InVivo | Anti-mouse PD-L1 (B7-H1) (10F.2H11)-InVivo | Anti-mouse PD-L1 (B7-H1) (10F.2H11)-InVivo reacts with mouse PD-L1 (programmed death ligand 1) also known as B7-H1 or CD274. The 10F.2H11 antibody has been shown to sterically and functionally block PD-L1/CD80 (B7-1) interactions and does not block PD-L1/PD-1 interactions. | |
| A2176New | Anti-mouse CD8α (53-6.7)-Invivo | in vivo CD8+ T cell depletion | ||
| A2136 | Anti-mouse CD40-InVivo | Anti-mouse CD40-InVivo | Anti-mouse CD40-InVivo reacts with mouse CD40 also known as Bp50. CD40 is a type I transmembrane glycoprotein that belongs to the tumor necrosis factor receptor (TNFR) superfamily. | |
| A2137 | Anti-mouse CD8β (Lyt 3.2)-InVivo | Anti-mouse CD8β (Lyt 3.2)-InVivo | Anti-mouse CD8β (Lyt 3.2)-InVivo reacts with mouse CD8β also known as Lyt 3.2. The CD8 antigen is a transmembrane glycoprotein that acts as a co-receptor for the T cell receptor (TCR). | |
| A2138 | Anti-mouse IL-2-InVivo | Anti-mouse IL-2-InVivo | Anti-mouse IL-2-InVivo reacts with mouse IL-2, a cytokine that is mainly produced by T cells in response to antigenic or mitogenic stimulation. | |
| A2177New | Anti-mouse IFNγR (CD119)-Invivo | in vivo/vitro IFNγR neutralization | ||
| A2140 | Anti-mouse/human/rat CD47 (IAP)-InVivo | Anti-mouse/human/rat CD47 (IAP)-InVivo | Anti-mouse/human/rat CD47 (IAP)-InVivo reacts with mouse CD47 otherwise known as integrin-associated protein (IAP). CD47 is an approximately 50 kDa glycosylated five transmembrane protein that is ubiquitously expressed by both hematopoietic cells such as T and B lymphocytes, monocytes, platelets and erythrocytes and non-hematopoietic cells. | |
| A2178New | Anti-mouse/human IL-5-Invivo | in vivo IL-5 neutralization, in vivo eosinophil depletion | ||
| A2175New | Anti-mouse CD11c-Invivo | in vivo targeting of dendritic cells |
