
- 阻害剤
- 研究分野別
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- 化合物ライブラリー
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-II
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at info@selleck.co.jp to customize your library.
You could select:
- 抗体
- 新製品
- お問い合わせ
Aurora Kinase
Aurora Kinase製品
- All (40)
- Aurora Kinase阻害剤 (40)
- 新製品
| 製品コード | 製品名称 | 製品説明 | 文献中Selleckの製品使用例 | お客様のフィードバック |
|---|---|---|---|---|
| S1460 | SP600125 | SP600125(Nsc75890)は、JNK1、JNK2、JNK3に対する広範なJNK阻害剤であり、細胞フリーアッセイでのIC50はそれぞれ40nM、40nM、90nMです。MKK4に対して10倍、MKK3、MKK6、PKB、PKCαに対して25倍、ERK2、p38、Chk1、EGFRなどに対して100倍高い選択性を示します。SP600125は、Aurora kinase A、FLT3、TRKAを含むserine/threonine kinasesの広範な阻害剤でもあり、IC50はそれぞれ60nM、90nM、70nMです。SP600125はautophagyを阻害し、apoptosisを活性化します。 |

|
|
| S1133 | Alisertib (MLN8237) | Alisertib (MLN8237)は、無細胞アッセイにおいてIC50が1.2 nMの選択的Aurora A阻害剤です。Aurora BよりもAurora Aに対して200倍以上の選択性を示します。Alisertibは細胞周期停止、apoptosis、およびautophagyを誘導します。現在治験第3相です。 |
-S113303W0120130926.gif)
|
|
| S1147 | Barasertib-HQPA (AZD2811) | Defosbarasertib (AZD1152-HQPA, AZD2811, INH-34, Barasertib-HQPA) は、高選択的なAurora B阻害剤で、in vitroアッセイにおけるIC50値は0.37 nMです。Aurora AよりもAurora Bに対して約3700倍高い選択性を示します。第1相。 |

|
|
| S1048 | Tozasertib (VX-680) | Tozasertib (VX-680)は、Aurora Aに対するKiappがセルフリーアッセイで0.6 nMのパンAurora阻害剤であり、Aurora B/Aurora Cに対する効力は低く、他の55のキナーゼよりもAurora Aに対して100倍選択的です。唯一の例外は、Kiが30 nMでTozasertibによって阻害されるFms-related tyrosine kinase-3 (FLT-3)とBCR-ABL tyrosine kinaseです。Tozasertibはapoptosisとautophagyを誘導します。フェーズ2。 |
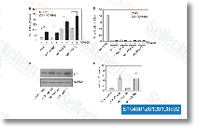
|
|
| S1103 | ZM 447439 | ZM 447439は、Aurora AとAurora Bに対する選択的かつATP競合的阻害剤であり、それぞれ110 nMと130 nMのIC50を示します。MEK1、Src、LckよりもAurora A/Bに対して8倍以上の選択性があり、CDK1/2/4、Plk1、Chk1などに対してはほとんど影響を与えません。 |
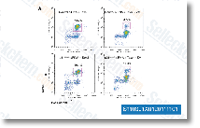
|
|
| S1529 | Hesperadin | Hesperadinは、セルフリーアッセイにおいて250 nMのIC50でAurora Bを強力に阻害します。AMPK、Lck、MKK1、MAPKAP-K1、CHK1、およびPHKの活性を著しく低下させますが、in vivoではMKK1活性を阻害しません。 |
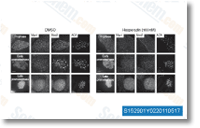
|
|
| S1100 | MLN8054 | MLN8054は、Sf9昆虫細胞においてIC50が4nMの、強力かつ選択的なAurora A阻害剤です。Aurora BよりもAurora Aに対して40倍以上の選択性を示します。フェーズ1。 |
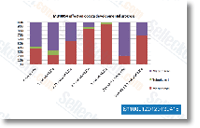
|
|
| S1107 | Danusertib (PHA-739358) | Danusertib (PHA-739358)は、細胞を含まないアッセイでAurora A/B/Cに対するIC50が13 nM/79 nM/61 nMのAurora Kinase阻害剤であり、Abl、TrkA、c-RET、FGFR1に対して中程度の効力を持ち、Lck、VEGFR2/3、c-Kit、CDK2などに対してはより低い効力を持つ。Danusertibはapoptosis、細胞周期停止、およびautophagyを誘導する。フェーズ2。 |
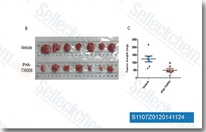
|
|
| S2770 | MK-5108 | MK-5108は、無細胞アッセイにおいて0.064 nMのIC50を持つ高選択的なAurora A阻害剤であり、Aurora B/Cに対しては220倍および190倍の選択性を示しますが、TrkAに対しては100倍未満の選択性で阻害します。MK-5108(VX-689)はautophagyを誘導します。フェーズ1。 |
-S277001W0220130927.gif)
|
|
| S1134 | AT9283 | AT9283は、in vitroアッセイにおいてIC50が1.2 nM/1.1 nMの強力なJAK2/3阻害剤であり、Aurora A/BおよびAbl1(T315I)にも強力に作用します。 |

|
|
| S1451 | TCS7010 (Aurora A Inhibitor I) | TCS7010 (Aurora A Inhibitor I) は、3.4 nMのIC50値を示す、新規かつ強力なAurora Aの選択的阻害剤です。Aurora Bと比較して、Aurora Aに対する選択性は1000倍高いです。Aurora A Inhibitor I (TC-S 7010) は、ROSを介したUPRシグナル伝達経路を介してapoptosisを誘発します。 |
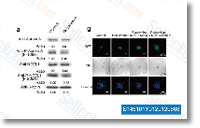
|
|
| S1249 | JNJ-7706621 | JNJ-7706621は、IC50が9 nM/4 nMでCDK1/2に最高の効力を持つパン-CDK阻害剤であり、セルフリーアッセイではCDK3/4/6よりもCDK1/2に対して>6倍の選択性を示します。また、Aurora A/Bを強力に阻害し、Plk1およびWee1には活性がありません。 |
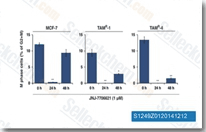
|
|
| S2719 | AMG-900 | AMG 900 は、Aurora A/B/C に対する強力かつ高選択的な pan-Aurora kinases 阻害剤であり、IC50 は 5 nM/4 nM /1 nM です。p38α、Tyk2、JNK2、Met、Tie2 よりも Aurora kinases に対して 10 倍以上の選択性を示します。フェーズ 1。 |
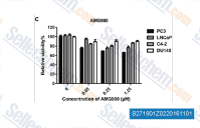
|
|
| S7588 | Reversine | Reversineは、ヒトA3アデノシン受容体の強力なアンタゴニストであり、Ki値は0.66 μMです。また、パンオーロラA/B/Cキナーゼ阻害剤でもあり、それぞれのIC50値は400 nM/500 nM/400 nMです。幹細胞の脱分化にも使用されます。 |
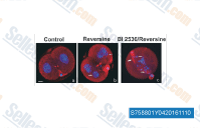
|
|
| S1454 | PHA-680632 | PHA-680632は、Aurora A、Aurora B、およびAurora Cの強力な阻害剤であり、それぞれのIC50は27 nM、135 nM、120 nMです。FGFR1、FLT3、LCK、PLK1、STLK2、VEGFR2/3に対するIC50は10~200倍高いです。 |
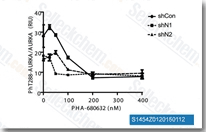
|
|
| S2744 | CCT137690 | CCT137690 は、Aurora A、Aurora B、Aurora C の高選択的阻害剤であり、IC50 はそれぞれ 15 nM、25 nM、19 nM です。hERG イオンチャネルにはほとんど影響を与えません。 |
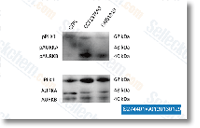
|
|
| S2158 | KW-2449 | KW-2449は、IC50が6.6 nMで主にFLT3を標的とする多標的阻害剤であり、FGFR1、Bcr-Abl、Aurora Aに対して中程度の効力があります。PDGFRβ、IGF-1R、EGFRにはほとんど影響がありません。第1相。 |
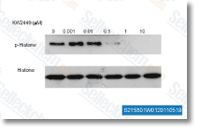
|
|
| S2740 | GSK1070916 | GSK1070916は、Aurora B/Cの可逆的ATP競合阻害剤であり、IC50は3.5 nM/6.5 nMです。これは、密接に関連するAurora A-TPX2複合体に対して100倍以上の選択性を示します。フェーズ1。 |

|
|
| S1154 | SNS-314 | SNS-314メシル酸塩は、Aurora A、Aurora B、Aurora Cの強力かつ選択的な阻害剤であり、それぞれのIC50値は9 nM、31 nM、3 nMです。Trk A/B、Flt4、Fms、Axl、c-Raf、DDR2に対する効力はそれほど高くありません。第1相。 |

|
|
| S2718 | TAK-901 | TAK-901は、IC50が21nM/15nMであるAurora A/Bの新規阻害剤です。細胞性JAK2、c-Src、またはAblの強力な阻害剤ではありません。フェーズ1。 | ||
| S1181 | ENMD-2076 | ENMD-2076は、Aurora AおよびFlt3に対し選択的な活性を持ち、IC50値はそれぞれ14 nMおよび1.86 nMです。Aurora BよりもAurora Aに対して25倍選択的であり、RET、SRC、NTRK1/TRKA、CSF1R/FMS、VEGFR2/KDR、FGFR、PDGFRαに対してはより低効力です。ENMD-2076は、広範囲のヒト固形腫瘍および造血器癌細胞株の増殖を0.025〜0.7 μMのIC50で阻害し、apoptosisおよびG2/M期停止を誘導します。フェーズ2。 |

|
|
| S1171 | CYC116 | CYC116はAurora A/Bの強力な阻害剤であり、Kiは8.0 nM/9.2 nMで、VEGFR2(Kiは44 nM)に対する効力は低く、CDKに比べて50倍高い効力を持ち、PKA、Akt/PKB、PKCに対しては活性がなく、GSK-3α/β、CK2、Plk1、SAPK2Aには影響を与えません。フェーズ1。 |

|
|
| S1051New | AZD1152(Barasertib) | AZD1152は、Aurora Bの選択的阻害剤であり、Kiは0.36 nMです。 | ||
| S7843 | BI-847325 | BI-847325は、経口投与可能で選択的なデュアルMEK/Auroraキナーゼ阻害剤であり、Xenopus laevis Aurora B、ヒトAurora A、Aurora C、およびヒトMEK1とMEK2に対するIC50値はそれぞれ3 nM、25 nM、15 nM、25 nM、および4 nMです。第1相。 | ||
| S8699 | SNS-314 Mesylate | SNS-314 Mesylateは、Aurora A、Aurora B、Aurora Cに対する強力かつ選択的な阻害剤であり、IC50値はそれぞれ9 nM、31 nM、3 nMです。また、Trk A/B、Flt4、Fms、Axl、c-Raf、DDR2に対しては、より低い効力しか持ちません。 | ||
| S8782 | LY3295668 | LY3295668 (AK-01) は、Aurora A キナーゼの強力で経口活性のある特異的阻害剤であり、AURKA および AURKB に対する Ki はそれぞれ 0.8 nM および 1038 nM です。 | ||
| S1519 | CCT129202 | CCT129202は、Aurora A、Aurora B、Aurora Cに対するATP競合型pan-Aurora阻害剤であり、それぞれのIC50は0.042 µM、0.198 µM、0.227 µMです。FGFR3、GSK3β、PDGFRβなどに対する効力は低いです。 |

|
|
| S9658 | SP-96 | SP-96は、IC50が0.316 nMの強力で選択的な非ATP競合性のAurora B阻害剤です。SP-96は、トリプルネガティブ乳がん(TNBC)の研究に使用できます。 | ||
| S6214 | H-1152 dihydrochloride | H-1152 dihydrochloride(2HCl)は、膜透過性のある選択的なRho関連プロテインキナーゼ(ROCK)阻害剤です。H-1152は、ROCK2、PKA、PKC、PKG、AuroraA、CaMK2をそれぞれIC50値0.0120 µM、3.03 µM、5.68 µM、0.360 µM、0.745 µM、0.180 µMで阻害します。 | ||
| S7065 | MK-8745 | MK-8745は、Aurora Aの強力かつ選択的阻害剤であり、IC50は0.6 nMで、Aurora BよりもAurora Aに対して450倍以上の選択性を示します。 | ||
| S2018 | ENMD-2076 L-(+)-Tartaric acid | ENMD-2076 L-(+)-酒石酸は、ENMD-2076の酒石酸であり、Aurora AおよびFlt3に対し、IC50が14 nMおよび1.86 nMの選択的活性を示し、Aurora BよりもAurora Aに対して25倍選択的であり、VEGFR2/KDR、VEGFR3、FGFR1、FGFR2、PDGFRαに対する効力は低い。第2相。 |

|
|
| S9741 | TAS-119 | TAS-119は、Aurora Aを含むAurora Bに対し、それぞれIC50が1.0 nMおよび95 nMである、強力で選択的かつ経口活性型の阻害剤です。抗腫瘍活性を示します。 | ||
| E0338 | AKI603 | AKI-603は、BCR-ABL-T315I変異によって媒介される抵抗性を克服するために開発されたAuroraキナーゼA(AurA)の阻害剤です。 | ||
| E0342 | Aurora kinase Inhibitor II | Aurora kinase Inhibitor IIは、強力かつ選択的なATP競合阻害剤であるアニリノキナゾリンであり、細胞透過性があり、細胞周期、特に細胞分裂の調節に関与しています。 | ||
| E0616 | Chiauranib | Chiauranib(CS2164)は、複数のキナーゼターゲットであるオーロラBキナーゼ(AURKB)、コロニー刺激因子1受容体(CSF1R)、血管内皮増殖因子受容体(VEGFR)/血小板由来増殖因子受容体(PDGFR)/c-Kitを選択的に阻害することで、腫瘍細胞の急速な増殖を抑制し、抗腫瘍免疫を増強し、腫瘍血管新生を阻害し、抗腫瘍効果を発揮します。 | ||
| E5904New | 6K465 | 6K465 is a pyrimidine-based inhibitor of Aurora A, that reduces levels of c-MYC and N-MYC oncoproteins. It demonstrates compelling antitumor efficacy. | ||
| S2931 | Aurora Kinase Inhibitor III | Aurora Kinase Inhibitor IIIは、IC50が42nMのAurora A kinaseの強力な阻害剤であり、BMX、BTK、IGF-1R、c-Src、TRKB、SYK、およびEGFR(IC50sはそれぞれ386、3,550、591、1,980、2,510、887、および>10,000nM)に対してAurora Aの高い選択性を示します。 | ||
| E1651 | CD532 | CD532 は、45 nM の IC50 を持つ強力な Aurora A キナーゼ阻害剤です。Aurora-A の本来のコンフォメーションを破壊し、N-Myc 駆動型癌における N-Myc の分解を促進し、抗増殖効果も示します。 | ||
| S1272 | XL228 | XL228は、野生型ABLキナーゼ、ABL T315I、Aurora A、IGF-1R、SRC、およびLYNに対して、それぞれ5 nM、1.4 nM、3.1 nM、1.6 nM、6.1 nM、2 nMのIC50値を持つプロテインキナーゼ阻害剤です。 | ||
| E1435 | Tinengotinib | Tinengotinib (TT-00420) は、IC50値がそれぞれ1.2/3.3 nMでAurora A/Bを強力に阻害する新規多重キナーゼ阻害剤です。また、FGFR1/2/3、VEGFR1、JAK1/2、およびCSF1Rに対して強力な阻害活性を示し、トリプルネガティブ乳がん(TNBC)のいくつかの主要なシグナル伝達経路の治療に使用できます。 | ||
| S1460 | SP600125 | SP600125(Nsc75890)は、JNK1、JNK2、JNK3に対する広範なJNK阻害剤であり、細胞フリーアッセイでのIC50はそれぞれ40nM、40nM、90nMです。MKK4に対して10倍、MKK3、MKK6、PKB、PKCαに対して25倍、ERK2、p38、Chk1、EGFRなどに対して100倍高い選択性を示します。SP600125は、Aurora kinase A、FLT3、TRKAを含むserine/threonine kinasesの広範な阻害剤でもあり、IC50はそれぞれ60nM、90nM、70nMです。SP600125はautophagyを阻害し、apoptosisを活性化します。 |

|
|
| S1133 | Alisertib (MLN8237) | Alisertib (MLN8237)は、無細胞アッセイにおいてIC50が1.2 nMの選択的Aurora A阻害剤です。Aurora BよりもAurora Aに対して200倍以上の選択性を示します。Alisertibは細胞周期停止、apoptosis、およびautophagyを誘導します。現在治験第3相です。 |
-S113303W0120130926.gif)
|
|
| S1147 | Barasertib-HQPA (AZD2811) | Defosbarasertib (AZD1152-HQPA, AZD2811, INH-34, Barasertib-HQPA) は、高選択的なAurora B阻害剤で、in vitroアッセイにおけるIC50値は0.37 nMです。Aurora AよりもAurora Bに対して約3700倍高い選択性を示します。第1相。 |

|
|
| S1048 | Tozasertib (VX-680) | Tozasertib (VX-680)は、Aurora Aに対するKiappがセルフリーアッセイで0.6 nMのパンAurora阻害剤であり、Aurora B/Aurora Cに対する効力は低く、他の55のキナーゼよりもAurora Aに対して100倍選択的です。唯一の例外は、Kiが30 nMでTozasertibによって阻害されるFms-related tyrosine kinase-3 (FLT-3)とBCR-ABL tyrosine kinaseです。Tozasertibはapoptosisとautophagyを誘導します。フェーズ2。 |

|
|
| S1103 | ZM 447439 | ZM 447439は、Aurora AとAurora Bに対する選択的かつATP競合的阻害剤であり、それぞれ110 nMと130 nMのIC50を示します。MEK1、Src、LckよりもAurora A/Bに対して8倍以上の選択性があり、CDK1/2/4、Plk1、Chk1などに対してはほとんど影響を与えません。 |

|
|
| S1529 | Hesperadin | Hesperadinは、セルフリーアッセイにおいて250 nMのIC50でAurora Bを強力に阻害します。AMPK、Lck、MKK1、MAPKAP-K1、CHK1、およびPHKの活性を著しく低下させますが、in vivoではMKK1活性を阻害しません。 |

|
|
| S1100 | MLN8054 | MLN8054は、Sf9昆虫細胞においてIC50が4nMの、強力かつ選択的なAurora A阻害剤です。Aurora BよりもAurora Aに対して40倍以上の選択性を示します。フェーズ1。 |

|
|
| S1107 | Danusertib (PHA-739358) | Danusertib (PHA-739358)は、細胞を含まないアッセイでAurora A/B/Cに対するIC50が13 nM/79 nM/61 nMのAurora Kinase阻害剤であり、Abl、TrkA、c-RET、FGFR1に対して中程度の効力を持ち、Lck、VEGFR2/3、c-Kit、CDK2などに対してはより低い効力を持つ。Danusertibはapoptosis、細胞周期停止、およびautophagyを誘導する。フェーズ2。 |

|
|
| S2770 | MK-5108 | MK-5108は、無細胞アッセイにおいて0.064 nMのIC50を持つ高選択的なAurora A阻害剤であり、Aurora B/Cに対しては220倍および190倍の選択性を示しますが、TrkAに対しては100倍未満の選択性で阻害します。MK-5108(VX-689)はautophagyを誘導します。フェーズ1。 |
-S277001W0220130927.gif)
|
|
| S1134 | AT9283 | AT9283は、in vitroアッセイにおいてIC50が1.2 nM/1.1 nMの強力なJAK2/3阻害剤であり、Aurora A/BおよびAbl1(T315I)にも強力に作用します。 |

|
|
| S1451 | TCS7010 (Aurora A Inhibitor I) | TCS7010 (Aurora A Inhibitor I) は、3.4 nMのIC50値を示す、新規かつ強力なAurora Aの選択的阻害剤です。Aurora Bと比較して、Aurora Aに対する選択性は1000倍高いです。Aurora A Inhibitor I (TC-S 7010) は、ROSを介したUPRシグナル伝達経路を介してapoptosisを誘発します。 |

|
|
| S1249 | JNJ-7706621 | JNJ-7706621は、IC50が9 nM/4 nMでCDK1/2に最高の効力を持つパン-CDK阻害剤であり、セルフリーアッセイではCDK3/4/6よりもCDK1/2に対して>6倍の選択性を示します。また、Aurora A/Bを強力に阻害し、Plk1およびWee1には活性がありません。 |

|
|
| S2719 | AMG-900 | AMG 900 は、Aurora A/B/C に対する強力かつ高選択的な pan-Aurora kinases 阻害剤であり、IC50 は 5 nM/4 nM /1 nM です。p38α、Tyk2、JNK2、Met、Tie2 よりも Aurora kinases に対して 10 倍以上の選択性を示します。フェーズ 1。 |

|
|
| S7588 | Reversine | Reversineは、ヒトA3アデノシン受容体の強力なアンタゴニストであり、Ki値は0.66 μMです。また、パンオーロラA/B/Cキナーゼ阻害剤でもあり、それぞれのIC50値は400 nM/500 nM/400 nMです。幹細胞の脱分化にも使用されます。 |

|
|
| S1454 | PHA-680632 | PHA-680632は、Aurora A、Aurora B、およびAurora Cの強力な阻害剤であり、それぞれのIC50は27 nM、135 nM、120 nMです。FGFR1、FLT3、LCK、PLK1、STLK2、VEGFR2/3に対するIC50は10~200倍高いです。 |

|
|
| S2744 | CCT137690 | CCT137690 は、Aurora A、Aurora B、Aurora C の高選択的阻害剤であり、IC50 はそれぞれ 15 nM、25 nM、19 nM です。hERG イオンチャネルにはほとんど影響を与えません。 |

|
|
| S2158 | KW-2449 | KW-2449は、IC50が6.6 nMで主にFLT3を標的とする多標的阻害剤であり、FGFR1、Bcr-Abl、Aurora Aに対して中程度の効力があります。PDGFRβ、IGF-1R、EGFRにはほとんど影響がありません。第1相。 |

|
|
| S2740 | GSK1070916 | GSK1070916は、Aurora B/Cの可逆的ATP競合阻害剤であり、IC50は3.5 nM/6.5 nMです。これは、密接に関連するAurora A-TPX2複合体に対して100倍以上の選択性を示します。フェーズ1。 |

|
|
| S1154 | SNS-314 | SNS-314メシル酸塩は、Aurora A、Aurora B、Aurora Cの強力かつ選択的な阻害剤であり、それぞれのIC50値は9 nM、31 nM、3 nMです。Trk A/B、Flt4、Fms、Axl、c-Raf、DDR2に対する効力はそれほど高くありません。第1相。 |

|
|
| S2718 | TAK-901 | TAK-901は、IC50が21nM/15nMであるAurora A/Bの新規阻害剤です。細胞性JAK2、c-Src、またはAblの強力な阻害剤ではありません。フェーズ1。 | ||
| S1181 | ENMD-2076 | ENMD-2076は、Aurora AおよびFlt3に対し選択的な活性を持ち、IC50値はそれぞれ14 nMおよび1.86 nMです。Aurora BよりもAurora Aに対して25倍選択的であり、RET、SRC、NTRK1/TRKA、CSF1R/FMS、VEGFR2/KDR、FGFR、PDGFRαに対してはより低効力です。ENMD-2076は、広範囲のヒト固形腫瘍および造血器癌細胞株の増殖を0.025〜0.7 μMのIC50で阻害し、apoptosisおよびG2/M期停止を誘導します。フェーズ2。 |

|
|
| S1171 | CYC116 | CYC116はAurora A/Bの強力な阻害剤であり、Kiは8.0 nM/9.2 nMで、VEGFR2(Kiは44 nM)に対する効力は低く、CDKに比べて50倍高い効力を持ち、PKA、Akt/PKB、PKCに対しては活性がなく、GSK-3α/β、CK2、Plk1、SAPK2Aには影響を与えません。フェーズ1。 |

|
|
| S1051New | AZD1152(Barasertib) | AZD1152は、Aurora Bの選択的阻害剤であり、Kiは0.36 nMです。 | ||
| S7843 | BI-847325 | BI-847325は、経口投与可能で選択的なデュアルMEK/Auroraキナーゼ阻害剤であり、Xenopus laevis Aurora B、ヒトAurora A、Aurora C、およびヒトMEK1とMEK2に対するIC50値はそれぞれ3 nM、25 nM、15 nM、25 nM、および4 nMです。第1相。 | ||
| S8699 | SNS-314 Mesylate | SNS-314 Mesylateは、Aurora A、Aurora B、Aurora Cに対する強力かつ選択的な阻害剤であり、IC50値はそれぞれ9 nM、31 nM、3 nMです。また、Trk A/B、Flt4、Fms、Axl、c-Raf、DDR2に対しては、より低い効力しか持ちません。 | ||
| S8782 | LY3295668 | LY3295668 (AK-01) は、Aurora A キナーゼの強力で経口活性のある特異的阻害剤であり、AURKA および AURKB に対する Ki はそれぞれ 0.8 nM および 1038 nM です。 | ||
| S1519 | CCT129202 | CCT129202は、Aurora A、Aurora B、Aurora Cに対するATP競合型pan-Aurora阻害剤であり、それぞれのIC50は0.042 µM、0.198 µM、0.227 µMです。FGFR3、GSK3β、PDGFRβなどに対する効力は低いです。 |

|
|
| S9658 | SP-96 | SP-96は、IC50が0.316 nMの強力で選択的な非ATP競合性のAurora B阻害剤です。SP-96は、トリプルネガティブ乳がん(TNBC)の研究に使用できます。 | ||
| S6214 | H-1152 dihydrochloride | H-1152 dihydrochloride(2HCl)は、膜透過性のある選択的なRho関連プロテインキナーゼ(ROCK)阻害剤です。H-1152は、ROCK2、PKA、PKC、PKG、AuroraA、CaMK2をそれぞれIC50値0.0120 µM、3.03 µM、5.68 µM、0.360 µM、0.745 µM、0.180 µMで阻害します。 | ||
| S7065 | MK-8745 | MK-8745は、Aurora Aの強力かつ選択的阻害剤であり、IC50は0.6 nMで、Aurora BよりもAurora Aに対して450倍以上の選択性を示します。 | ||
| S2018 | ENMD-2076 L-(+)-Tartaric acid | ENMD-2076 L-(+)-酒石酸は、ENMD-2076の酒石酸であり、Aurora AおよびFlt3に対し、IC50が14 nMおよび1.86 nMの選択的活性を示し、Aurora BよりもAurora Aに対して25倍選択的であり、VEGFR2/KDR、VEGFR3、FGFR1、FGFR2、PDGFRαに対する効力は低い。第2相。 |

|
|
| S9741 | TAS-119 | TAS-119は、Aurora Aを含むAurora Bに対し、それぞれIC50が1.0 nMおよび95 nMである、強力で選択的かつ経口活性型の阻害剤です。抗腫瘍活性を示します。 | ||
| E0338 | AKI603 | AKI-603は、BCR-ABL-T315I変異によって媒介される抵抗性を克服するために開発されたAuroraキナーゼA(AurA)の阻害剤です。 | ||
| E0342 | Aurora kinase Inhibitor II | Aurora kinase Inhibitor IIは、強力かつ選択的なATP競合阻害剤であるアニリノキナゾリンであり、細胞透過性があり、細胞周期、特に細胞分裂の調節に関与しています。 | ||
| E0616 | Chiauranib | Chiauranib(CS2164)は、複数のキナーゼターゲットであるオーロラBキナーゼ(AURKB)、コロニー刺激因子1受容体(CSF1R)、血管内皮増殖因子受容体(VEGFR)/血小板由来増殖因子受容体(PDGFR)/c-Kitを選択的に阻害することで、腫瘍細胞の急速な増殖を抑制し、抗腫瘍免疫を増強し、腫瘍血管新生を阻害し、抗腫瘍効果を発揮します。 | ||
| E5904New | 6K465 | 6K465 is a pyrimidine-based inhibitor of Aurora A, that reduces levels of c-MYC and N-MYC oncoproteins. It demonstrates compelling antitumor efficacy. | ||
| S2931 | Aurora Kinase Inhibitor III | Aurora Kinase Inhibitor IIIは、IC50が42nMのAurora A kinaseの強力な阻害剤であり、BMX、BTK、IGF-1R、c-Src、TRKB、SYK、およびEGFR(IC50sはそれぞれ386、3,550、591、1,980、2,510、887、および>10,000nM)に対してAurora Aの高い選択性を示します。 | ||
| E1651 | CD532 | CD532 は、45 nM の IC50 を持つ強力な Aurora A キナーゼ阻害剤です。Aurora-A の本来のコンフォメーションを破壊し、N-Myc 駆動型癌における N-Myc の分解を促進し、抗増殖効果も示します。 | ||
| S1272 | XL228 | XL228は、野生型ABLキナーゼ、ABL T315I、Aurora A、IGF-1R、SRC、およびLYNに対して、それぞれ5 nM、1.4 nM、3.1 nM、1.6 nM、6.1 nM、2 nMのIC50値を持つプロテインキナーゼ阻害剤です。 | ||
| E1435 | Tinengotinib | Tinengotinib (TT-00420) は、IC50値がそれぞれ1.2/3.3 nMでAurora A/Bを強力に阻害する新規多重キナーゼ阻害剤です。また、FGFR1/2/3、VEGFR1、JAK1/2、およびCSF1Rに対して強力な阻害活性を示し、トリプルネガティブ乳がん(TNBC)のいくつかの主要なシグナル伝達経路の治療に使用できます。 | ||
| S1051New | AZD1152(Barasertib) | AZD1152は、Aurora Bの選択的阻害剤であり、Kiは0.36 nMです。 | ||
| E5904New | 6K465 | 6K465 is a pyrimidine-based inhibitor of Aurora A, that reduces levels of c-MYC and N-MYC oncoproteins. It demonstrates compelling antitumor efficacy. |
