- 阻害剤
- 研究分野別
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- 化合物ライブラリー
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-II
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at info@selleck.co.jp to customize your library.
You could select:
- 抗体
- 新製品
- お問い合わせ
Cisplatin
別名:NSC 119875, Cisplatinum, cis-diamminedichloroplatinum II, CDDP, cis DDP, DDP
シスプラチン (Cisplatin (NSC 119875, Cisplatinum, cis-diamminedichloroplatinum II, CDDP, cis DDP, DDP)) は無機白金錯体であり、腫瘍細胞中において DNA 付加物を形成することにより DNA 合成を阻害する能力を有します。シスプラチンはフェロトーシス (ferroptosis) を活性化し、オートファジー (autophagy) を誘導します。溶液は都度調製してください。Solutions are unstable and should be fresh-prepared.DMSO is not recommended to dissolve platinum-based drugs, which can easily lead to drug inactivation.
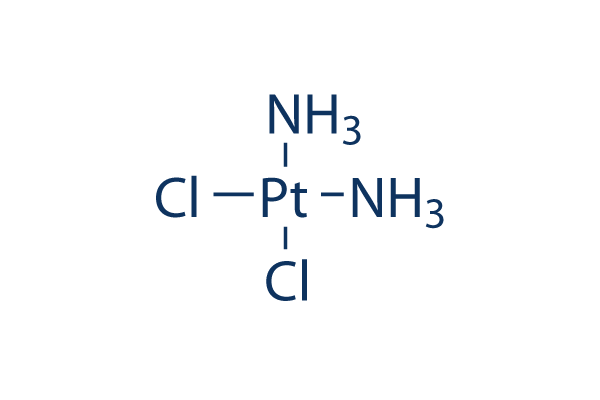
CAS No. 15663-27-1
文献中Selleckの製品使用例(831)
製品安全説明書
現在のバッチを見る:
純度:
99.84%
99.84
Cisplatinと併用されることが多い化合物
Explores the combined effect that potentiates the anti-tumor effects of this compound to resolve ATM-deficient non-small cell lung cancer in vivo
Berzosertib (VX-970), when given in combination with it, enhances the efficacy of this compound in patient-derived lung tumour xenografts.
Combination treatment with BAY 1895344 and this compound achieved strong synergistic activity on the proliferation of human HT-29 colorectal cancer cells in vitro.
Cisplatin関連製品
シグナル伝達経路
DNA/RNA Synthesis阻害剤の選択性比較
Cell Data
| Cell Lines | Assay Type | Concentration | Incubation Time | 活性情報 | PMID |
|---|---|---|---|---|---|
| OVC cells (A2780, TOV-112D, and cis-A2780) | Cell Cytotoxicity Assay | 0.5, 1, 2.5, 5, 10, 20, and 50 μM | 48 h | Combination of cisplatin and MEK inhibitor cobimetinib (10 nM) enhances cell death in three ovarian cancer cell lines (A2780, TOV-112D, and cis-A2780). | 31057611 |
| Human osteosarcoma cells (HOS, 143B, U2OS and MG‑63) | Cell cycle analysis | 2 μM | 48 h | Cisplatin treatment markedly increased the G2/M population in all cell lines. | 31059083 |
| HCC cell lines HepG2 and Huh7 | Cell viability assay | 0-30 μM | 48 h | CD133+ HCC cells exhibit resistance to cisplatin. | 31056532 |
| Saos-2 cells | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Saos-2 cells | 29435139 | |||
| OHS-50 cells | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for OHS-50 cells | 29435139 | |||
| SK-N-MC cells | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-MC cells | 29435139 | |||
| 他の多くの細胞株試験データをご覧になる場合はこちらをクリックして下さい | |||||
生物活性
| 製品説明 | シスプラチン (Cisplatin (NSC 119875, Cisplatinum, cis-diamminedichloroplatinum II, CDDP, cis DDP, DDP)) は無機白金錯体であり、腫瘍細胞中において DNA 付加物を形成することにより DNA 合成を阻害する能力を有します。シスプラチンはフェロトーシス (ferroptosis) を活性化し、オートファジー (autophagy) を誘導します。溶液は都度調製してください。Solutions are unstable and should be fresh-prepared.DMSO is not recommended to dissolve platinum-based drugs, which can easily lead to drug inactivation. | |
|---|---|---|
| 特性 | One of the most widely used and most potent chemotherapeutic agents. This product is not recommended to be dissolved in dimethylsulfoxide (DMSO). | |
| Targets |
|
| In Vitro | ||||
| In vitro |
Cisplatin induces cytotoxic by interaction with DNA to form DNA adducts which activate several signal transduction pathways, including Erk, p53, p73, and MAPK, which culminates in the activation of apoptosis. This compound (30 μM) treated for 6 h induces an apparent activation of Erk in HeLa cells, which is sustained over the following 14 h period. It also shows an effective antineoplastic activity by inducing tumor cells death. It displays ability to cause renal proximal tubular cell (RPTC) apoptosis, causing cell shrinkage, a 50-fold increase in caspase 3 activity, a 4-fold increase in phosphatidylserine externalization, and 5- and 15-fold increases in chromatin condensation and DNA hypoploidy, respectively. This chemical (800 μM) causes typical features of necrosis of RPTC after treatment for 4 hr. |
|||
|---|---|---|---|---|
| 細胞実験 | 細胞株 | Leukemia L1210/0 cells | ||
| 濃度 | 7 μg/mL | |||
| 反応時間 | 2 hours | |||
| 実験の流れ | L1210/0 cells are maintained in an exponential suspension culture at 37 ℃ in a humidified atmosphere of 5% CO2 in McCoy's medium 5a (modified), supplemented with 15% calfserum, and Fungizone. L1210/0 cells are incubated in Cisplatin (7 μg/mL) for 2 hr at 37 ℃. To measure growth inhibition, the cells are centrifuged, washed once, resuspended in fresh medium at 30 × 103 to 50 × 103 cells/mL, and incubated for 3 days. Cell numbers are determined on a Coulter Counter. An aliquot of cells is diluted with an equal volume of 0.4% trypan blue. Viability is recorded as the percentage of cells that has excluded trypan blue. Cells incubated with this compound as above are also diluted into 0.1% agar and allowed to grow for 2 weeks when colonies are counted. |
|||
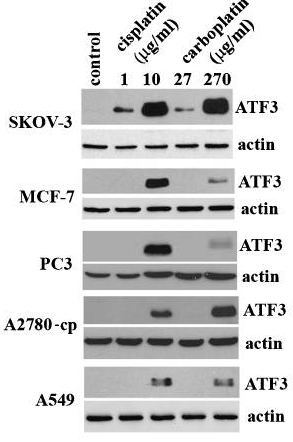
| 実験結果図 | Methods | Biomarkers | 結果図 | PMID |
| Western blot | ATF3 FEN1 PD-L1 / p-MEK / MEK / p-STAT3 / STAT3 LC3B-I / LC3B-II / Beclin-1 p-AMPK / AMPK / p-mTOR / mTOR |
|
20651982 | |
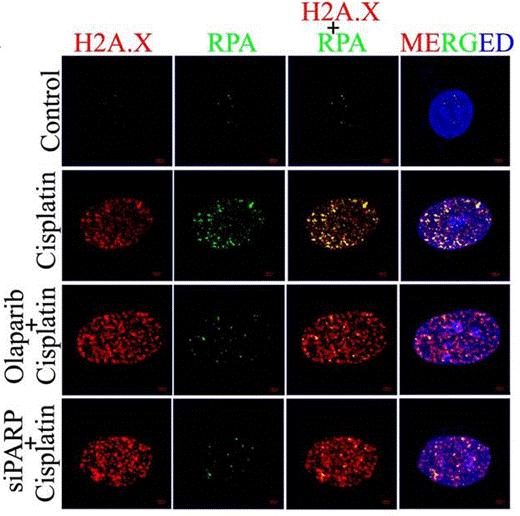
| Immunofluorescence | H2A.X / RPA γ-H2A.X / 53BP1 N-cadherin / E-cadherin / Vimentin LC3B |
|
28993682 | |
| Growth inhibition assay | Cell viability |
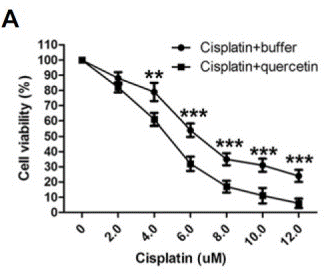
|
26062553 | |
| In Vivo | ||
| In Vivo |
Cisplatin has been demonstrated to be efficient in regression tumor growth in a wide variety of animal tumors models, including head and neck cancer xenografts, cervical squamous carcinoma xenografts, testicular carcinoma xenografts, ovarian cancer xenografts, breast carcinoma xenografts, colonic carcinoma, heterotransplanted hepatoblastoma, and so on. This compound (5 mg/kg) given weekly i.v. at the day 1 and 7 induces a tumor growth inhibition (GI) of 77.5% and 85.1% of the serous xenografts Ov.Ri(C) and OVCAR-3, respectively. |
|
|---|---|---|
| 動物実験 | 動物モデル | Female NMRI/Cpb (nuinu) mice |
| 投与量 | 5 mg/kg | |
| 投与経路 | i.v. | |
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06356155 | Not yet recruiting | Urothelial Carcinoma |
University of Michigan Rogel Cancer Center |
October 2024 | Phase 2 |
| NCT06393816 | Not yet recruiting | Large Cell Neuroendocrine Carcinoma of the Lung |
Centre Leon Berard|Groupe Français de Pneumo-Cancérologie |
May 2024 | Phase 2 |
| NCT06406465 | Not yet recruiting | Carcinoma Neuroendocrine|Tumor Neuroendocrine|Tumors Neuroendocrine|Neuroendocrine; Carcinoma|Small Cell; Receptors |
National Cancer Institute (NCI)|National Institutes of Health Clinical Center (CC) |
May 15 2024 | Phase 2 |
| NCT04915183 | Recruiting | Hearing Loss|Head and Neck Cancer |
National Institute on Deafness and Other Communication Disorders (NIDCD)|National Institutes of Health Clinical Center (CC) |
May 15 2024 | Phase 2 |
|
化学情報
| 分子量 | 300.05 | 化学式 | Cl2H6N2Pt |
| CAS No. | 15663-27-1 | SDF | Download Cisplatin SDFをダウンロードする |
| Smiles | [NH2-].[NH2-].Cl[Pt+2]Cl | ||
| 保管 | 2 years 4°C(in the dark) powder | 溶液状態は不安定なので使用直前に調整してください。少量づつ分包して保管し、都度使い切る事が推奨されます。 | |
|
In vitro |
DMF : 15 mg/mL Water : Insoluble Ethanol : Insoluble |
モル濃度計算器 |
|
in vivo Add solvents to the product individually and in order. |
投与溶液組成計算機 | |||||
実験計算
投与溶液組成計算機(クリア溶液)
ステップ1:実験データを入力してください。(実験操作によるロスを考慮し、動物数を1匹分多くして計算・調製することを推奨します)
mg/kg
g
μL
匹
ステップ2:投与溶媒の組成を入力してください。(ロット毎に適した溶解組成が異なる場合があります。詳細については弊社までお問い合わせください)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
計算結果:
投与溶媒濃度: mg/ml;
DMSOストック溶液調製方法: mg 試薬を μL DMSOに溶解する(濃度 mg/mL, 注:濃度が当該ロットのDMSO溶解度を超える場合はご連絡ください。 )
投与溶媒調製方法:Take μL DMSOストック溶液に μL PEG300,を加え、完全溶解後μL Tween 80,を加えて完全溶解させた後 μL ddH2O,を加え完全に溶解させます。
投与溶媒調製方法:μL DMSOストック溶液に μL Corn oil,を加え、完全溶解。
注意:1.ストック溶液に沈殿、混濁などがないことをご確認ください;
2.順番通りに溶剤を加えてください。次のステップに進む前に溶液に沈殿、混濁などがないことを確認してから加えてください。ボルテックス、ソニケーション、水浴加熱など物理的な方法で溶解を早めることは可能です。
技術サポート
ストックの作り方、阻害剤の保管方法、細胞実験や動物実験の際に注意すべき点など、製品を取扱う時に問い合わせが多かった質問に対しては取扱説明書でお答えしています。
他に質問がある場合は、お気軽にお問い合わせください。
* 必須
よくある質問(FAQ)
質問1:
What is the appropriate concentration of DMF for cell culture and animal study?
回答
It depends on the cell type. The final concentration of DMF should be better limited to less than 0.1% if possible, or below 1%. Using saline as a vehicle for it at up to 3mg/ml is recommended. It's a suspension and can be administrated via oral gavage.