- 阻害剤
- 研究分野別
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- 化合物ライブラリー
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-II
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at info@selleck.co.jp to customize your library.
You could select:
- 抗体
- 新製品
- お問い合わせ
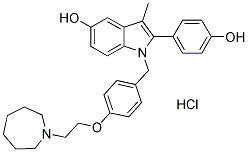
Bazedoxifene (WAY-140424) HCl
別名:TSE-424
Bazedoxifene HCl (WAY-140424、TSE-424)は、新規の非ステロイド性インドールベースのエストロゲン受容体モジュレーター(SERM)であり、ERαおよびERβの両方に結合し、それぞれのIC50は23 nMおよび89 nMです。
CAS No. 198480-56-7
文献中Selleckの製品使用例(17)
カスタマーフィードバック2例
製品安全説明書
現在のバッチを見る:
純度:
99.06%
99.06
Bazedoxifene (WAY-140424) HCl関連製品
Estrogen/progestogen Receptor阻害剤の選択性比較
生物活性
| 製品説明 | Bazedoxifene HCl (WAY-140424、TSE-424)は、新規の非ステロイド性インドールベースのエストロゲン受容体モジュレーター(SERM)であり、ERαおよびERβの両方に結合し、それぞれのIC50は23 nMおよび89 nMです。 | ||||
|---|---|---|---|---|---|
| Targets |
|
| In Vitro | ||||
| In vitro | Bazedoxifene is a third generation selective estrogen receptor modulator (SERM). Bazedoxifene does not stimulate ERα mediated transcriptional activity and acts as an antagonist to estradiol in cultured breast cancer (bMCF-7) cells. Similar results are seen in other cell lines including CHO (ovarian), HepG2 (hepatic) or GTI-7 (neuronal) with bazedoxifene having no ERα agonist activity and acting as an antagonist to estradiol action. Bazedoxifene does not stimulate proliferation of MCF-7 cells but did inhibit 17β-estradiol-induced proliferation with IC50 of 0.19 nM. | |||
|---|---|---|---|---|
| Kinase Assay | Ligand binding competition experiments | |||
| Test compounds are initially solubilized in DMSO and the final concentration of DMSO in the binding assay is ≤ 1%. Eight dilutions of each test compound are used as an unlabelled competitor for [3H]17β-estradiol. Typically, a set of compound dilutions would be tested simultaneously on human, rat and mouse ER-α and ER-β. The results are plotted as measured DPM vs. concentration of test compound. For dose-response curve fitting, a four parameter logistic model on the transformed, weighted data are fit and the IC50 is defined as the concentration of compound decreasing maximum [3H]estradiol binding by 50%. For active compounds, the IC50 is determined at least three times. It should be noted that IC50 values are not direct measures of a ligand’s affinity for the receptor. Rather, they can only be compared as relative values, in this case to 17β-estradiol. | ||||
| 細胞実験 | 細胞株 | MCF-7 | ||
| 濃度 | ~10 nM | |||
| 反応時間 | 7 days | |||
| 実験の流れ | For the proliferation assay, cells are plated at 20,000 cells/well in a 24-well plate in DMEM/F12 (50:50) (phenol red-free) with 10% charcoal/dextran-treated FBS and 1 × GlutaMAX-1. After overnight incubation, the medium is aspirated and treatments in DMEM/F12 (50:50) (phenol red-free) with 2% charcoal/dextran-treated FBS and 1 × GlutaMAX-1 are added to the wells. Each plate has a vehicle (baseline proliferation) and treatments. Treatments included 10 pM 17β-estradiol determined to be the EC80 for 17β-estradiol and 17β-estradiol in combination with six concentrations of BZA. Treatments from d 1 are renewed on d 3 and d 6 by aspirating medium from wells and replacing with fresh medium and treatments. On d 7, cells are detached from the plate using trypsin-EDTA and counted using a Multisizer II. | |||
| In Vivo | ||
| In Vivo | In an immature rat model, bazedoxifene increases uterine wet weight 35% at a dose of 0.5 mg/kg compared to an 85% increase with raloxifene at the same dose and a 300% increase in uterine weight with ethinyl estradiol at a dose of 10 μg/kg. Ovarectomized rats treated with 0.3 mg/d bazedoxifene displayed maintenance of bone mass and bone strength similar to effects seen with 2 μg/d ethinyl estradiol, 3 mg/d raloxifene, or sham operated animals. | |
|---|---|---|
| 動物実験 | 動物モデル | Sprague Dawley rats |
| 投与量 | 0.5 and 5.0 mg/kg | |
| 投与経路 | SC | |
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT03475719 | Unknown status | Osteoporosis Postmenopausal |
Huons Co. Ltd. |
January 11 2018 | Phase 1 |
| NCT03005340 | Unknown status | Healthy |
Alvogen Korea |
December 2016 | Phase 1 |
| NCT02729701 | Completed | Breast Cancer |
University of Kansas Medical Center|Pfizer |
May 2016 | Phase 2 |
| NCT01973738 | Unknown status | Selective Estrogen Receptor Modulator |
Toshihiko Kono|Tomidahama Hospital |
January 2012 | -- |
|
化学情報
| 分子量 | 507.06 | 化学式 | C30H34N2O3.HCl |
| CAS No. | 198480-56-7 | SDF | -- |
| Smiles | CC1=C(N(C2=C1C=C(C=C2)O)CC3=CC=C(C=C3)OCCN4CCCCCC4)C5=CC=C(C=C5)O.Cl | ||
| 保管 | |||
|
In vitro |
DMSO : 90 mg/mL ( (177.49 mM); 吸湿したDMSOは溶解度を減少させます。新しいDMSOをご使用ください。) Water : Insoluble Ethanol : Insoluble |
モル濃度計算器 |
|
in vivo Add solvents to the product individually and in order. |
投与溶液組成計算機 | |||||
実験計算
投与溶液組成計算機(クリア溶液)
ステップ1:実験データを入力してください。(実験操作によるロスを考慮し、動物数を1匹分多くして計算・調製することを推奨します)
mg/kg
g
μL
匹
ステップ2:投与溶媒の組成を入力してください。(ロット毎に適した溶解組成が異なる場合があります。詳細については弊社までお問い合わせください)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
計算結果:
投与溶媒濃度: mg/ml;
DMSOストック溶液調製方法: mg 試薬を μL DMSOに溶解する(濃度 mg/mL, 注:濃度が当該ロットのDMSO溶解度を超える場合はご連絡ください。 )
投与溶媒調製方法:Take μL DMSOストック溶液に μL PEG300,を加え、完全溶解後μL Tween 80,を加えて完全溶解させた後 μL ddH2O,を加え完全に溶解させます。
投与溶媒調製方法:μL DMSOストック溶液に μL Corn oil,を加え、完全溶解。
注意:1.ストック溶液に沈殿、混濁などがないことをご確認ください;
2.順番通りに溶剤を加えてください。次のステップに進む前に溶液に沈殿、混濁などがないことを確認してから加えてください。ボルテックス、ソニケーション、水浴加熱など物理的な方法で溶解を早めることは可能です。
技術サポート
ストックの作り方、阻害剤の保管方法、細胞実験や動物実験の際に注意すべき点など、製品を取扱う時に問い合わせが多かった質問に対しては取扱説明書でお答えしています。
他に質問がある場合は、お気軽にお問い合わせください。
* 必須