- 阻害剤
- 研究分野別
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- 化合物ライブラリー
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-II
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at info@selleck.co.jp to customize your library.
You could select:
- 抗体
- 新製品
- お問い合わせ
RAD001 (Everolimus)
別名:RAD001,SDZ-RAD
エベロリムス (Everolimus (RAD001, SDZ-RAD)) は FKBP12 に結合することで形成した複合体により mTOR を阻害します。Cell-free assay における IC50 は1.6 ~ 2.4 nM です。エベロリムスは アポトーシス (apoptosis) と オートファジー (autophagy) を誘発することによりがん細胞の増殖を阻害します。
CAS No. 159351-69-6
文献中Selleckの製品使用例(760)
製品安全説明書
現在のバッチを見る:
純度:
99.78%
99.78
RAD001 (Everolimus)関連製品
シグナル伝達経路
mTOR阻害剤の選択性比較
Cell Data
| Cell Lines | Assay Type | Concentration | Incubation Time | 活性情報 | PMID |
|---|---|---|---|---|---|
| A549 | Autophagy assay | 5 uM | 6 h | Induction of autophagy in human A549 cells assessed as increase in conversion of LC3-1 to LC3-2 at 5 uM after 6 hrs by Western blot analysis | 26176165 |
| A549 | Function Assay | 5 uM | 3 to 24 h | Inhibition of mTOR phosphorylation at ser2448 in human A549 cells at 5 uM after 3 to 24 hrs by Western blot analysis | 26176165 |
| A549 | Function Assay | 5 uM | 3 to 24 h | Inhibition of mTOR in human A549 cells assessed as downregulation of p70S6K phosphorylation at thr389 at 5 uM after 3 to 24 hrs by Western blot analysis | 26176165 |
| OVCAR3 | Cytotoxic Assay | 72 h | IC50=16 μM | 24445311 | |
| DU145 | Cytotoxic Assay | 72 h | IC50=8 μM | 24445311 | |
| SK-HEP1 | Cytotoxic Assay | 72 h | IC50=12 μM | 24445311 | |
| CAKI1 | Cytotoxic Assay | 72 h | IC50=14 μM | 24445311 | |
| HT29 | Cytotoxic Assay | 72 h | IC50=15 μM | 24445311 | |
| HCT116 | Cytotoxic Assay | 72 h | IC50=12 μM | 24445311 | |
| ColoR | Cytotoxic Assay | 72 h | IC50=8.7 μM | 24445311 | |
| Colo205 | Cytotoxic Assay | 72 h | IC50=20 μM | 24445311 | |
| SQ20B | Cytotoxic Assay | 72 h | IC50=5.5 μM | 24445311 | |
| HOP62 | Cytotoxic Assay | 72 h | IC50=19 μM | 24445311 | |
| Colo205 | Function Assay | 24 h | Inhibits mTORC1 in human COLO205 cells assessed as reduction of S6 phosphorylation at 0.1 to 8 uM | 24836070 | |
| Colo205 | Function Assay | 24 h | Inhibits mTORC1 in human COLO205 cells assessed as reduction of 4-EBP1 phosphorylation at 0.1 to 8 uM | 24836070 | |
| SK-HEP1 | Function Assay | 24 h | Inhibits mTORC1 in human SK-HEP1 cells assessed as reduction of S6 phosphorylation at 0.1 to 8 uM | 24836070 | |
| SK-HEP1 | Function Assay | 24 h | Inhibits mTORC1 in human SK-HEP1 cells assessed as reduction of 4-EBP1 phosphorylation at 0.1 to 8 uM | 24836070 | |
| Sf9 | Function Assay | 15 to 20 mins | Inhibition of human BSEP overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-taurocholate in presence of ATP measured after 15 to 20 mins by membrane vesicle transport assay, IC50 = 2 μM. | 23956101 | |
| Sf9 | Function Assay | 20 mins | Inhibition of human MRP2 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and GSH measured after 20 mins by membrane vesicle transport assay, IC50 = 11.3 μM. | 23956101 | |
| 他の多くの細胞株試験データをご覧になる場合はこちらをクリックして下さい | |||||
生物活性
| 製品説明 | エベロリムス (Everolimus (RAD001, SDZ-RAD)) は FKBP12 に結合することで形成した複合体により mTOR を阻害します。Cell-free assay における IC50 は1.6 ~ 2.4 nM です。エベロリムスは アポトーシス (apoptosis) と オートファジー (autophagy) を誘発することによりがん細胞の増殖を阻害します。 | ||||
|---|---|---|---|---|---|
| Targets |
|
| In Vitro | ||||
| In vitro | Everolimus exhibits the immunosuppressive activity which is comparable to that of rapamycin. This compound competes with immobilized FK 506 for binding to biotinylated FKBP12 and shows the inhibitory effect on a two-way MLR performed with spleen cells from BALB/c and CBA mice with IC50 of 0.12-1.8 nM. It also shows antiangiogenic/vascular effects in VEGF-induced HUVEC proliferation with IC50 of 0.12 nM and bFGF-induced HUVEC proliferation with IC50 of 0.8 nM, respectively. A recent study shows that this chemical shows a dose-dependent inhibitory effects on both the total cells and the stem cells from the BT474 cell line and the primary breast cancer cells with IC50 of 156 nM in total cells of primary breast cancer cells and 71 nM in total cells of BT474 cells. In addition, combination treatment with this compound and trastuzumab produces the significantly increased inhibition on the growth of cancer stem cells with the inhibition rate increased by more than 50 %. | |||
|---|---|---|---|---|
| Kinase Assay | FKBP12 binding assay & Mixed lymphocyte reaction (MLR) | |||
| FKBP12 binding assay: Binding to the FK 506 binding protein (FKBP12) is indirectly assessed by means of an ELISA-type competition assay. FK 506 is included in each individual experiment as a standard, and the inhibitory activity is expressed as relative IC50 compared to FK 506 (rIC50 = IC50 of this compound/IC50 FK 506). Mixed lymphocyte reaction (MLR): The immunosuppressive activities of RAP and its derivatives are assessed in a two-way MLR, using spleen cells of BALB/c and CBA mice. RAP is included in each individual experiment as a standard, and the inhibitory activity is expressed as relative IC50 compared to RAP (rIC50 = IC50 of this compound/IC50 RAP). | ||||
| 細胞実験 | 細胞株 | BT474 cell line and the primary breast cancer cells | ||
| 濃度 | 0.001-10 μM | |||
| 反応時間 | 24 hours | |||
| 実験の流れ | The 3-(4-5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide dye reduction assay (MTT assay) is used to compare the effects of Everolimus or trastuzumab on total breast cancer cells and breast CSCs. The total cells and the stem cells from the BT474 cell line and the primary breast cancer cells are respectively seeded into 96-well plates with different concentrations of the drugs, with five wells for each concentration, and the cells are cultured at 37 °C with 5 % CO2 in an incubator for 24 hours. The concentrations of this compound are 1 nM, 10 nM, 100 nM, 1 μM and 10 μM, and the concentrations of trastuzumab are 0.5 μg/mL, 1 μg/mL, 10 μg/mL, 50 μg/mL, and 100 μg/mL. The combinatorial inhibitory effect of this compound and Trastuzumab on the in vitro growth of breast CSCs is examined by MTT assay as well using 10 μg/mL trastuzumab in combination of increasing concentrations of this chemical (1 nM, 10 nM, 100 nM and 1 μM). After drug treatment for 24 hours, 20 μL MTT [5 mg/mL in phosphate buffered saline (PBS)] is added to each well, and the cells are incubated at 37 °C with 5 % CO2 and saturated humidity for 4 hours. Following the subsequent removal of the supernatant, 150 μL dimethyl sulfoxide (DMSO) is added to each well, and the cells are vortexed for 10 minutes. The light absorbance (OD value) is measured for each well using an ELISA reader. Each experiment is repeated in triplicate, and dose–response curves are plotted. The probit software of the statistical software package SPSS 17.0 for Windows is used to calculate the inhibitory concentration (IC50) of this compound. | |||
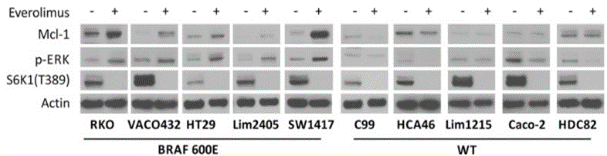
| 実験結果図 | Methods | Biomarkers | 結果図 | PMID |
| Western blot | Mcl-1 / p-ERK / S6K1(T389) pS6RP cleaved caspase-3 |
|
27351224 | |
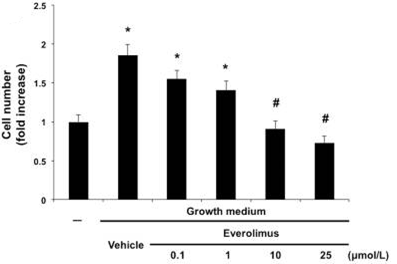
| Growth inhibition assay | Cell counts IC50 Cell proliferation |
|
27127803 | |
| Immunofluorescence | TERT |
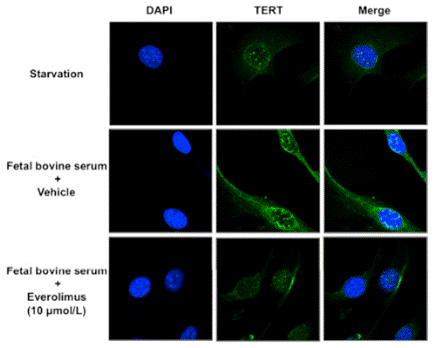
|
27127803 | |
| In Vivo | ||
| In Vivo | Everolimus (0.1 to 10 mg/kg) dose-dependently inhibits growth of the primary (ear) and lymph node metastases of B16/BL6 melanoma, with decreased total number of vessels and reduced mature vessels. In a xenograft animal model of BT474 stem cells, this compound shows significant reductions in mean tumor sizes (590.6 mm3), compared to the control group with a tumor size of 698 mm3. Furthermore, combination treatment with this compound and trastuzumab significantly decreases the xenograft tumor size (410.8 mm3) more than this compound treatment alone. | |
|---|---|---|
| 動物実験 | 動物モデル | Cultured BT474 stem cells are injected beneath the left breast pad of BALB/c nude mice. |
| 投与量 | ≤2 mg/kg | |
| 投与経路 | Administered via p.o. | |
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05843253 | Not yet recruiting | High Grade Glioma|Diffuse Intrinsic Pontine Glioma|Anaplastic Astrocytoma|Glioblastoma|Glioblastoma Multiforme|Diffuse Midline Glioma H3 K27M-Mutant|Metastatic Brain Tumor|WHO Grade III Glioma|WHO Grade IV Glioma |
Nationwide Children''s Hospital|Novartis |
May 30 2024 | Phase 2 |
| NCT05501769 | Active not recruiting | Breast Cancer |
Arvinas Estrogen Receptor Inc.|Pfizer|Arvinas Inc. |
September 8 2022 | Phase 1 |
| NCT05476939 | Recruiting | Diffuse Intrinsic Pontine Glioma|Diffuse Midline Glioma H3 K27M-Mutant |
Gustave Roussy Cancer Campus Grand Paris|Chimerix|Innovative Therapies For Children with Cancer Consortium |
September 29 2022 | Phase 3 |
| NCT05293964 | Recruiting | Breast Cancer |
Jiangsu Simcere Pharmaceutical Co. Ltd. |
May 18 2022 | Phase 1 |
|
化学情報
| 分子量 | 958.22 | 化学式 | C53H83NO14 |
| CAS No. | 159351-69-6 | SDF | Download RAD001 (Everolimus) SDFをダウンロードする |
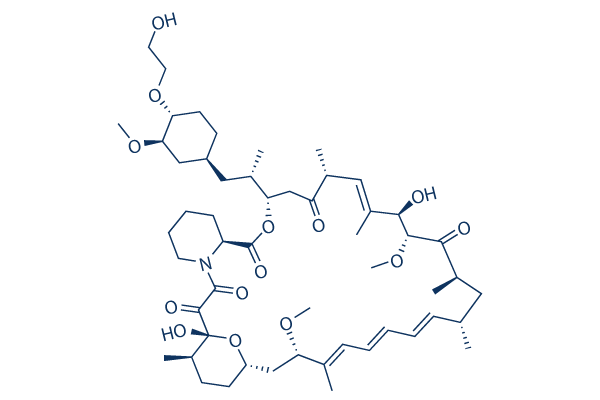
| Smiles | CC1CCC2CC(C(=CC=CC=CC(CC(C(=O)C(C(C(=CC(C(=O)CC(OC(=O)C3CCCCN3C(=O)C(=O)C1(O2)O)C(C)CC4CCC(C(C4)OC)OCCO)C)C)O)OC)C)C)C)OC | ||
| 保管 | |||
|
In vitro |
DMSO : 100 mg/mL ( (104.36 mM); 吸湿したDMSOは溶解度を減少させます。新しいDMSOをご使用ください。) Ethanol : 100 mg/mL Water : Insoluble |
モル濃度計算器 |
|
in vivo Add solvents to the product individually and in order. |
投与溶液組成計算機 | |||||
実験計算
投与溶液組成計算機(クリア溶液)
ステップ1:実験データを入力してください。(実験操作によるロスを考慮し、動物数を1匹分多くして計算・調製することを推奨します)
mg/kg
g
μL
匹
ステップ2:投与溶媒の組成を入力してください。(ロット毎に適した溶解組成が異なる場合があります。詳細については弊社までお問い合わせください)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
計算結果:
投与溶媒濃度: mg/ml;
DMSOストック溶液調製方法: mg 試薬を μL DMSOに溶解する(濃度 mg/mL, 注:濃度が当該ロットのDMSO溶解度を超える場合はご連絡ください。 )
投与溶媒調製方法:Take μL DMSOストック溶液に μL PEG300,を加え、完全溶解後μL Tween 80,を加えて完全溶解させた後 μL ddH2O,を加え完全に溶解させます。
投与溶媒調製方法:μL DMSOストック溶液に μL Corn oil,を加え、完全溶解。
注意:1.ストック溶液に沈殿、混濁などがないことをご確認ください;
2.順番通りに溶剤を加えてください。次のステップに進む前に溶液に沈殿、混濁などがないことを確認してから加えてください。ボルテックス、ソニケーション、水浴加熱など物理的な方法で溶解を早めることは可能です。
技術サポート
ストックの作り方、阻害剤の保管方法、細胞実験や動物実験の際に注意すべき点など、製品を取扱う時に問い合わせが多かった質問に対しては取扱説明書でお答えしています。
他に質問がある場合は、お気軽にお問い合わせください。
* 必須
よくある質問(FAQ)
質問1:
For the in vivo work, I know it needs to be dissolved in 30% propylene glycol (dilution in water) and 5% Tween 80. Would the final solution be a clear liquid or a turbid suspension?
回答
Our S1120 in 30% Propylene glycol+5% Tween 80+ddH2O at 5mg/ml is a clear solution. And for oral gavage, there is another common vehicle, 1% CMC Na. It can be dissolved in it at 30mg/ml as a suspension.