
- 阻害剤
- 研究分野別
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- 化合物ライブラリー
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-II
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at info@selleck.co.jp to customize your library.
You could select:
- 抗体
- 新製品
- お問い合わせ
Staurosporine (STS)
別名:AM-2282,Antibiotic AM-2282
スタウロスポリン (Staurosporine (AM-2282、Antiviotic AM-2282、STS)) は、PKCα、PKCγ、および PKCη に対する強力な PKC 阻害剤であり、IC50 は 2 nM、5 nM、および 4 nM です。 無細胞アッセイでは、PKCδ (20 nM)、PKCε (73 nM) に対して効力が低く、PKCζ (1086 nM) に対してほとんど活性がありません。 また、PKA、PKG、S6K、CaMKII などの他のキナーゼに対しても阻害活性を示します。臨床フェーズ3。
CAS No. 62996-74-1
文献中Selleckの製品使用例(189)
製品安全説明書
現在のバッチを見る:
純度:
99.61%
99.61
Staurosporine (STS)と併用されることが多い化合物
Staurosporine (STS)関連製品
Antineoplastic and Immunosuppressive Antibiotics阻害剤の選択性比較
Cell Data
| Cell Lines | Assay Type | Concentration | Incubation Time | 活性情報 | PMID |
|---|---|---|---|---|---|
| HepG2 cells | Cytotoxicity assay | 100 uM | 3 days | Cytotoxicity against human HepG2 cells at 100 uM after 3 days by CellTiter-Glo luminescent cell viability assay, IC50 = 0.018 μM. | 28993106 |
| Sf21 cells | Function assay | 30 uM | Inhibition of human CHK2 kinase expressed in insect Sf21 cells using phospho-CREBtide as substrate at 30 uM, IC50 = 0.027 μM. | 21334796 | |
| Sf21 cells | Function assay | 30 uM | Inhibition of human recombinant IKK-alpha expressed in insect Sf21 cells using phospho-Ulight-IkappaB-alpha as substrate at 30 uM, IC50 = 0.039 μM. | 21334796 | |
| Sf21 cells | Function assay | 30 uM | Inhibition of human recombinant IKK-beta expressed in insect Sf21 cells using phospho-Ulight-IkappaB-alpha as substrate at 30 uM, IC50 = 0.49 μM. | 21334796 | |
| A549 cells | Cytotoxicity assay | 72 h | Cytotoxicity against human A549 cells after 72 hrs by sulforhodamine B method, GI50 = 0.0024 μM. | 18484775 | |
| Sf9 cells | Function assay | 20 mins | Inhibition of human Fyn expressed in Sf9 cells after 20 mins by ELISA in presence of 100 umol/L ATP, IC50 = 0.51 μM. | 17315853 | |
| Sf9 cells | Function assay | 1 min | Inhibition of human Fyn expressed in Sf9 cells after 1 min by ELISA in presence of 100 umol/L ATP, IC50 = 0.27 μM. | 17315853 | |
| Sf9 cells | Function assay | 20 mins | Inhibition of human Fyn expressed in Sf9 cells after 20 mins by ELISA in presence of 10 umol/L ATP, IC50 = 0.083 μM. | 17315853 | |
| Sf9 cells | Function assay | 1 min | Inhibition of human Fyn expressed in Sf9 cells after 1 min by ELISA in presence of 10 umol/L ATP, IC50 = 0.036 μM. | 17315853 | |
| Sf9 cells | Function assay | 20 mins | Inhibition of human Fyn expressed in Sf9 cells after 20 mins by ELISA in presence of 1 umol/L ATP, IC50 = 0.015 μM. | 17315853 | |
| Sf9 cells | Function assay | 1 min | Inhibition of human Fyn expressed in Sf9 cells after 1 min by ELISA in presence of 1 umol/L ATP, IC50 = 0.005 μM. | 17315853 | |
| human PBMC | Function assay | 24 h | Suppression of IL2 production in human PBMC after 24 hrs by ELISA, IC50=16 nM. | 18585046 | |
| MDA-MB-231 cells | Cytotoxicity assay | 72 h | Cytotoxicity against human MDA-MB-231 cells after 72 hrs by sulforhodamine B method, GI50 = 0.0071 μM. | 18484775 | |
| HT29 cells | Cytotoxicity assay | 72 h | Cytotoxicity against human HT29 cells after 72 hrs by sulforhodamine B method, GI50 = 0.0109 μM. | 18484775 | |
| HT-29 cells | Apoptosis assay | 2 h | Induction of apoptosis in human HT-29 cells assessed as change in mitochondrial transmembrane potential after 2 hrs by JC1 staining based fluorescence assay, ED50 = 0.0026 μM. | 19422206 | |
| HUE cells | Function assay | 90 mins | Inhibition of VEGFR2 in HUE cells assessed as inhibition of VEGF-induced autophosphorylation treated for 90 mins before VEGF challenge by ELISA, IC50 = 0.07 μM. | 20170163 | |
| Sf9 cells | Function assay | 60 mins | Inhibition of human recombinant GST-fused NIK expressed in Sf9 cells after 60 mins by radiometric protein kinase assay, IC50 = 2.3 μM. | 20580552 | |
| HeLa cells | Cytotoxicity assay | 48 h | Cytotoxicity against human HeLa cells after 48 hrs by MTT assay, IC50 = 0.000004 μM. | 21388191 | |
| MCF7 cells | Cytotoxicity assay | 48 h | Cytotoxicity against human MCF7 cells after 48 hrs by MTT assay, IC50 = 0.05 μM. | 21388191 | |
| HT-29 cells | Function assay | 2 h | Effect on mitochondrial membrane potential in human HT-29 cells after 2 hrs using JC-1 staining by fluorescence assay, IC50 = 0.002 μM. | 21428375 | |
| HeLa cells | Cytotoxicity assay | 48 h | Cytotoxicity against human HeLa cells after 48 hrs by MTT assay, IC50 = 0.000004 μM. | 21488655 | |
| MCF7 cells | Cytotoxicity assay | 48 h | Cytotoxicity against human MCF7 cells after 48 hrs by MTT assay, IC50 = 0.05 μM. | 21488655 | |
| SF268 cells | Cytotoxicity assay | 48 h | Cytotoxicity against human SF268 cells after 48 hrs by SRB assay, GI50 = 0.044 μM. | 21513294 | |
| CHOK1 cells | Cytotoxicity assay | 48 h | Cytotoxicity against human CHOK1 cells after 48 hrs by SRB assay, GI50 = 0.13 μM. | 21513294 | |
| H460 cells | Cytotoxicity assay | 48 h | Cytotoxicity against human H460 cells after 48 hrs by SRB assay, GI50 = 3.6 μM. | 21513294 | |
| HT-29 cells | Cytotoxicity assay | 48 h | Cytotoxicity against human HT-29 cells after 48 hrs by SRB assay, GI50 = 3.6 μM. | 21513294 | |
| MCF7 cells | Cytotoxicity assay | 48 h | Cytotoxicity against human MCF7 cells after 48 hrs by SRB assay, GI50 = 11 μM. | 21513294 | |
| SW1116 cells | Function assay | 24 h | Inhibition of telomerase in human SW1116 cells after 24 hrs by TRAP-PCR-ELISA assay, IC50 = 8.32 μM. | 21962523 | |
| HT-29 cells | Apoptosis assay | 2 h | Induction of apoptosis in human HT-29 cells assessed reduction of mitochondrial membrane potential after 2 hrs by using JC1 staining by fluorescence cell-based assay, ED50 = 0.0026 μM. | 21973101 | |
| LoVo cells | Antiproliferative activity assay | 48 to 72 h | Antiproliferative activity against human LoVo cells after 48 to 72 hrs by MTT assay, IC50 = 0.001 μM. | 22182929 | |
| ST486 cells | Antiproliferative activity assay | 48 to 72 h | Antiproliferative activity against human ST486 cells after 48 to 72 hrs by MTT assay, IC50 = 0.007 μM. | 22182929 | |
| DLD1 cells | Antiproliferative activity assay | 48 to 72 h | Antiproliferative activity against human DLD1 cells after 48 to 72 hrs by MTT assay, IC50 = 0.009 μM. | 22182929 | |
| B16F10 cells | Antiproliferative activity assay | 48 h | Antiproliferative activity against mouse B16F10 cells after 48 hrs by MTT assay, IC50 = 2.82 μM. | 22503364 | |
| MCF7 cells | Antiproliferative activity assay | 48 h | Antiproliferative activity against human MCF7 cells after 48 hrs by MTT assay, IC50 = 3.07 μM. | 22503364 | |
| A431 cells | Cytotoxicity assay | 24 h | Cytotoxicity against human A431 cells after 24 hrs using Annexin VeFITC/propidium iodide staining by MTT assay, IC50 = 0.07 μM. | 22541051 | |
| MCF7 cells | Cytotoxicity assay | 24 h | Cytotoxicity against human MCF7 cells after 24 hrs using Annexin VeFITC/propidium iodide staining by MTT assay, IC50 = 0.18 μM. | 22541051 | |
| BJ cells | Cytotoxicity assay | 72 h | Cytotoxicity against human BJ cells after 72 hrs by Calcein AM assay, IC50 = 0.002 μM. | 22921081 | |
| CEM cells | Cytotoxicity assay | 72 h | Cytotoxicity against human CEM cells after 72 hrs by Calcein AM assay, IC50 = 0.023 μM. | 22921081 | |
| MCF7 cells | Cytotoxicity assay | 72 h | Cytotoxicity against human MCF7 cells after 72 hrs by Calcein AM assay, IC50 = 0.064 μM. | 22921081 | |
| HeLa cells | Cytotoxicity assay | 72 h | Cytotoxicity against human HeLa cells after 72 hrs by Calcein AM assay, IC50 = 0.175 μM. | 22921081 | |
| HepG2 cells | Function assay | 24 h | Inhibition of telomerase in human HepG2 cells after 24 hrs by TRAP-PCR-ELISA assay, IC50 = 4.14 μM. | 23018096 | |
| SW1116 cells | Antiproliferative activity assay | 48 h | Antiproliferative activity against human SW1116 cells after 48 hrs by MTT method, IC50 = 4.95 μM. | 23018096 | |
| HepG2 cells | Antiproliferative activity assay | 48 h | Antiproliferative activity against human HepG2 cells after 48 hrs by MTT method, IC50 = 6.73 μM. | 23018096 | |
| BGC823 cells | Antiproliferative activity assay | 48 h | Antiproliferative activity against human BGC823 cells after 48 hrs by MTT method, IC50 = 6.83 μM. | 23018096 | |
| HeLa cells | Antiproliferative activity assay | 48 h | Antiproliferative activity against human HeLa cells after 48 hrs by MTT method, IC50 = 9.12 μM. | 23018096 | |
| HepG2 cells | Function assay | 48 h | Inhibition of telomerase in human HepG2 cells after 48 hrs by TRAP-PCR-ELISA, IC50 = 8.3 μM. | 23279864 | |
| SW1116 cells | Function assay | 24 h | Inhibition of telomerase in human SW1116 cells after 24 hrs by TRAP-PCR-ELISA assay, IC50 = 4.18 μM. | 24286761 | |
| KE-97 cells | Cytotoxicity assay | 72 h | Cytotoxicity against human KE-97 cells after 72 hrs by CellTitre-Glo luminescent cell viability assay, IC50 = 0.13 μM. | 24328283 | |
| Jurkat cells | Cytotoxicity assay | 72 h | Cytotoxicity against human Jurkat cells after 72 hrs by CellTitre-Glo luminescent cell viability assay, IC50 = 0.14 μM. | 24328283 | |
| HuH7 cells | Cytotoxicity assay | 72 h | Cytotoxicity against human HuH7 cells after 72 hrs by CellTitre-Glo luminescent cell viability assay, IC50 = 0.23 μM. | 24328283 | |
| BGC823 cells | Cytotoxicity assay | 72 h | Cytotoxicity against human BGC823 cells after 72 hrs by CellTitre-Glo luminescent cell viability assay, IC50 = 0.38 μM. | 24328283 | |
| MCF7 cells | Cytotoxicity assay | 72 h | Cytotoxicity against human MCF7 cells after 72 hrs by CellTitre-Glo luminescent cell viability assay, IC50 = 0.52 μM. | 24328283 | |
| NIH/3T3 cells | Cytotoxicity assay | 96 h | Cytotoxicity against mouse NIH/3T3 cells after 96 hrs by SRB assay, IC50 = 0.2 μM. | 24361521 | |
| A2780 cells | Cytotoxicity assay | 96 h | Cytotoxicity against human A2780 cells after 96 hrs by SRB assay, IC50 = 0.2 μM. | 24361521 | |
| HT-29 cells | Cytotoxicity assay | 96 h | Cytotoxicity against human HT-29 cells after 96 hrs by SRB assay, IC50 = 0.2 μM. | 24361521 | |
| 8505C cells | Cytotoxicity assay | 96 h | Cytotoxicity against human 8505C cells after 96 hrs by SRB assay, IC50 = 0.2 μM. | 24361521 | |
| 518A2 cells | Cytotoxicity assay | 96 h | Cytotoxicity against human 518A2 cells after 96 hrs by SRB assay, IC50 = 0.2 μM. | 24361521 | |
| MCF7 cells | Cytotoxicity assay | 96 h | Cytotoxicity against human MCF7 cells after 96 hrs by SRB assay, IC50 = 0.4 μM. | 24361521 | |
| A549 cells | Cytotoxicity assay | 96 h | Cytotoxicity against human A549 cells after 96 hrs by SRB assay, IC50 = 0.6 μM. | 24361521 | |
| HEK293 cells | Cytotoxicity assay | 72 h | Cytotoxicity against HEK293 cells after 72 hrs by CellTiterGlo assay, IC50 = 0.056 μM. | 24763262 | |
| HeLa cells | Antiproliferative activity assay | 24 to 48 h | Antiproliferative activity against human HeLa cells after 24 to 48 hrs by CCK-8 assay, IC50 = 2.72 μM. | 24792811 | |
| A549 cells | Antiproliferative activity assay | 24 to 48 h | Antiproliferative activity against human A549 cells after 24 to 48 hrs by CCK-8 assay, IC50 = 3.05 μM. | 24792811 | |
| A549 cells | Cytotoxicity assay | 48 h | Cytotoxicity against human A549 cells after 48 hrs by MTT assay, IC50 = 0.02 μM. | 25825934 | |
| HeLa cells | Cytotoxicity assay | 48 h | Cytotoxicity against human HeLa cells after 48 hrs by MTT assay, IC50 = 0.025 μM. | 25825934 | |
| PC3 cells | Cytotoxicity assay | 48 h | Cytotoxicity against human PC3 cells after 48 hrs by MTT assay, IC50 = 0.031 μM. | 25825934 | |
| MGC-803 cells | Function assay | 24 h | Inhibition of telomerase in human MGC-803 cells after 24 hrs by TRAP-PCR-ELISA assay, IC50 = 8.97 μM. | 26900656 | |
| Sf9 insect cells | Function assay | 10 mins | Inhibition of N-terminal GST tagged recombinant full-length human CHK1 expressed in baculovirus Sf9 insect cells after 10 mins by luminescent ADP-Glo assay, IC50 = 0.0012 μM. | 27089211 | |
| Sf21 cells | Function assay | 60 mins | Inhibition of human recombinant JAK2 expressed in Sf21 cells assessed as reduction in Ulight-CAGAGAIETDKEYYTVKD phosphorylation pre-incubated before substrate addition and measured after 60 mins by LANCE detection method, IC50 = 0.002 μM. | 27137359 | |
| Neuro2a cells | Cytotoxicity assay | 72 h | Cytotoxicity against mouse Neuro2a cells assessed as cell viability after 72 hrs by MTT assay, IC50 = 0.0113 μM. | 28152427 | |
| MDA-MB-231 cells | Function assay | 16 h | Inhibition of retinoblastoma protein phosphorylation in human MDA-MB-231 cells after 16 hrs by Hoechst 33342 staining based fluorescence assay, IC50 = 0.066 μM. | 28431342 | |
| Sf9 insect cells | Function assay | 20 mins | Inhibition of recombinant human N-terminally GST-tagged EGFR L858R/T790M/C797S triple mutant expressed in baculovirus in Sf9 insect cells preincubated for 20 mins followed by addition of [33P]-ATP measured after 2 hrs by filter-binding method, IC50 = 0.00029 μM. | 28482151 | |
| HEK293 cells | Cytotoxicity assay | 72 h | Cytotoxicity against HEK293 cells assessed as decrease in cell viability after 72 hrs by CellTiter Glo luminescent assay, CC50 = 0.00354 μM. | 28624701 | |
| BT549 cells | Cytotoxicity assay | 24 h | Cytotoxic activity against human BT549 cells incubated for 24 hrs by MTT assay, IC50 = 0.08 μM. | 28705432 | |
| MDA-MB-468 cells | Cytotoxicity assay | 24 h | Cytotoxic activity against human MDA-MB-468 cells incubated for 24 hrs by MTT assay, IC50 = 0.15 μM. | 28705432 | |
| MDA-MB-231 cells | Cytotoxicity assay | 24 h | Cytotoxic activity against human MDA-MB-231 cells incubated for 24 hrs by MTT assay, IC50 = 0.24 μM. | 28705432 | |
| Vero cells | Cytotoxicity assay | 2 days | Cytotoxicity against African green monkey Vero cells assessed as reduction in cell viability after 2 days by cell-titer-glo luminescent assay, TC50 = 0.0024 μM. | 28763645 | |
| L929 cells | Antiproliferative activity assay | 5 days | Antiproliferative activity against mouse L929 cells after 5 days by WST-1 assay, IC50 = 0.2 μM. | 28956915 | |
| KB-3-1 cells | Antiproliferative activity assay | 5 days | Antiproliferative activity against human KB-3-1 cells after 5 days by WST-1 assay, IC50 = 0.2 μM. | 28956915 | |
| MCF7 cells | Antiproliferative activity assay | 5 days | Antiproliferative activity against human MCF7 cells after 5 days by WST-1 assay, IC50 = 1.8 μM. | 28956915 | |
| FS4-LTM cells | Antiproliferative activity assay | 24 h | Antiproliferative activity against human FS4-LTM cells after 24 hrs by WST-1 assay, IC50 = 2.8 μM. | 28956915 | |
| Vero cells | Cytotoxicity assay | 2 days | Cytotoxicity against African green monkey Vero cells after 2 days by CellTiter-Glo luminescent cell viability assay, IC50 = 0.0024 μM. | 28993106 | |
| NIH/3T3 cells | Cytotoxicity assay | 96 h | Cytotoxicity against mouse NIH/3T3 cells after 96 hrs by SRB assay, EC50 = 0.008 μM. | 29197730 | |
| 518A2 cells | Cytotoxicity assay | 96 h | Cytotoxicity against human 518A2 cells after 96 hrs by SRB assay, EC50 = 0.03 μM. | 29197730 | |
| A549 cells | Cytotoxicity assay | 96 h | Cytotoxicity against human A549 cells after 96 hrs by SRB assay, EC50 = 0.04 μM. | 29197730 | |
| MCF7 cells | Cytotoxicity assay | 96 h | Cytotoxicity against human MCF7 cells after 96 hrs by SRB assay, EC50 = 0.1 μM. | 29197730 | |
| A2780 cells | Cytotoxicity assay | 96 h | Cytotoxicity against human A2780 cells after 96 hrs by SRB assay, EC50 = 0.12 μM. | 29197730 | |
| HT-29 cells | Cytotoxicity assay | 96 h | Cytotoxicity against human HT-29 cells after 96 hrs by SRB assay, EC50 = 0.15 μM. | 29197730 | |
| insect cells | Function assay | 30 mins | Inhibition of recombinant human PDGFRbeta expressed in insect cells using Ulight-Poly GAT[EAY(1:1:1)]n as substrate after 30 mins by LANCE method, IC50 = 0.00076 μM. | 29549836 | |
| Sf9 insect cells | Function assay | 60 mins | Inhibition of recombinant human KDR expressed in Sf9 insect cells using Ulight-CAGAGAIETDKEYYTVKD as substrate after 60 mins by LANCE method, IC50 = 0.0021 μM. | 29549836 | |
| insect cells | Function assay | 10 mins | Inhibition of recombinant human Src expressed in insect cells using Ulight-Poly GAT[EAY(1:1:1)]n as substrate after 10 mins by LANCE method, IC50 = 0.005 μM. | 29549836 | |
| insect cells | Function assay | 15 mins | Inhibition of recombinant human CDK1/Cyclin B expressed in insect cells using Ulight-CFFKNIVTPRTPPPSQGK-amide as substrate after 15 mins by LANCE method, IC50 = 0.025 μM. | 29549836 | |
| insect cells | Function assay | 15 mins | Inhibition of recombinant human CDK1/Cyclin B expressed in insect cells using Ulight-CFFKNIVTPRTPPPSQGK-amide as substrate after 15 mins by LANCE method, IC50 = 0.025 μM. | 29549836 | |
| Sf9 insect cells | Function assay | 15 mins | Inhibition of recombinant human Flt1 expressed in Sf9 insect cells using Ulight-TK peptide as substrate after 15 mins by LANCE method, IC50 = 0.035 μM. | 29549836 | |
| Sf21 insect cells | Function assay | 15 mins | Inhibition of recombinant human Aurora-A expressed in Sf21 insect cells using Ulight-RRRSLLE as substrate after 15 mins by LANCE method, IC50 = 0.039 μM. | 29549836 | |
| insect cells | Function assay | 60 mins | Inhibition of recombinant human AKT1 expressed in insect cells using CREBtide as substrate after 60 mins by LANCE method, IC50 = 0.056 μM. | 29549836 | |
| Sf9 insect cells | Function assay | 60 mins | Inhibition of recombinant human FAK expressed in Sf9 insect cells using Ulight-TK peptide as substrate after 60 mins by LANCE method, IC50 = 0.062 μM. | 29549836 | |
| insect cells | Function assay | 60 mins | Inhibition of recombinant human ABL expressed in insect cells using Ulight-TK peptide as substrate after 60 mins by LANCE method, IC50 = 0.29 μM. | 29549836 | |
| insect cells | Function assay | 60 mins | Inhibition of recombinant human c-MET expressed in insect cells using Ulight-CAGAGAIETDKEYYTVKD as substrate after 60 mins by LANCE method, IC50 = 0.31 μM. | 29549836 | |
| PC3 cells | Cytotoxicity assay | 72 h | Cytotoxicity against human PC3 cells after 72 hrs by SRB assay, IC50 = 0.039 μM. | 29558119 | |
| Sf9 cells | Function assay | 45 mins | Inhibition of recombinant human C-terminal His-tagged/N-terminal GST-tagged EGFR (668 to 1210 residues) expressed in baculovirus infected Sf9 cells using Poly-(Glu, Tyr) as substrate measured after 45 minutes by Kinase-Glo luminescent assay, IC50 = 0.047 μM. | 29902719 | |
| Sf9 cells | Function assay | 45 mins | Inhibition of recombinant human N-terminal GST-tagged HER2 (679 to 1255 residues) expressed in baculovirus infected Sf9 cells using Poly-(Glu, Tyr) as substrate measured after 45 minutes by Kinase-Glo luminescent assay, IC50 = 0.0608 μM. | 29902719 | |
| HT-29 cells | Function assay | 3 h | Inhibition of mitochondrial membrane potential in human HT-29 cells after 3 hrs by JC-1 staining based fluorescence assay, IC50 = 0.02 μM. | 30057155 | |
| HCT116 cells | Cytotoxicity assay | 48 h | Cytotoxicity against human HCT116 cells assessed as decrease in cell viability after 48 hrs by MTT assay, IC50 = 0.03 μM. | 30106291 | |
| Huh7.5 cells | Cytotoxicity assay | 48 h | Cytotoxicity against human Huh7.5 cells assessed as decrease in cell viability after 48 hrs by MTT assay, IC50 = 0.6 μM. | 30106291 | |
| K562 cells | Cytotoxicity assay | 48 h | Cytotoxicity against human K562 cells assessed as decrease in cell viability after 48 hrs by MTT assay, IC50 = 1.7 μM. | 30106291 | |
| LO2 cells | Cytotoxicity assay | 48 h | Cytotoxicity against human LO2 cells assessed as decrease in cell viability after 48 hrs by MTT assay, IC50 = 9.5 μM. | 30106291 | |
| U937 cells | Cytotoxicity assay | Cytotoxicity against human U937 cells, IC50 = 2 μM. | 17088067 | ||
| Sf21 cells | Function assay | Inhibition of JAK3 expressed in Sf21 cells, IC50 = 0.006 μM. | 17088059 | ||
| FL5.12-Akt1 cells | Function assay | Activity against GSK3 phosphorylation in FL5.12-Akt1 cells, EC50 = 0.46 μM. | 16413780 | ||
| MiaPaCa-2 cells | Antiproliferative activity assay | Antiproliferative activity against human MiaPaCa-2 cells, IC50 = 0.37 μM. | 16413780 | ||
| FL5.12-Akt1 cells | Function assay | Activity against GSK3 in FL5.12-Akt1 cells, EC50 = 0.46 μM. | 16403626 | ||
| MiaPaCa-2 cells | Antiproliferative activity assay | Antiproliferative activity against MiaPaCa-2 cells by MTT assay, IC50 = 0.37 μM. | 16403626 | ||
| FL5.12-Akt1 cells | Antiproliferative activity assay | Antiproliferative activity against FL5.12-Akt1 cells by MTT assay, IC50 = 0.29 μM. | 16403626 | ||
| P19 cells | Function assay | Inhibition of Casein kinase 1 in P19 cells, IC50 = 1.4 μM. | 15771419 | ||
| P19 cells | Function assay | Inhibition of Insulin receptor kinase in P19 cells, IC50 = 0.2 μM. | 15771419 | ||
| HEK293 cells | Function assay | Inhibition of IL-8 release by HEK293 cells expressing PKC-beta2, IC50 = 0.077 μM. | 15771419 | ||
| P19 cells | Function assay | Inhibition of Vascular endothelial growth factor receptor in P19 cells, IC50 = 0.014 μM. | 15771419 | ||
| P19 cells | Function assay | Inhibition of Cyclin-dependent kinase 1 in P19 cells, IC50 = 0.008 μM. | 15771419 | ||
| P19 cells | Function assay | Inhibition of Calcium/calmodulin-dependent protein kinase type II in P19 cells, IC50 = 0.006 μM. | 15771419 | ||
| P19 cells | Function assay | Inhibition of Protein Kinase A in P19 cells, IC50 = 0.004 μM. | 15771419 | ||
| P19 cells | Function assay | Inhibition of Platelet-derived growth factor receptor in P19 cells, IC50 = 0.002 μM. | 15771419 | ||
| P19 cells | Function assay | Inhibition of Platelet-derived growth factor receptor in P19 cells, IC50 = 0.002 μM. | 15771419 | ||
| HEK293 cells | Function assay | Inhibition of PKC-beta II mediated IL-8 release from HEK293 cells by ELISA, IC50 = 0.077 μM. | 12941331 | ||
| DLD-1 cells | Antiproliferative activity assay | Antiproliferative activity against human colon cancer cell line (DLD-1 cells) using MTT assay, IC50 = 0.009 μM. | 11591505 | ||
| ST-486 cells | Antiproliferative activity assay | Antiproliferative activity against burkit lymphoma cell line (ST-486 cells) using MTT assay, IC50 = 0.007 μM. | 11591505 | ||
| umbilical vein endothelial cells | Antiproliferative activity assay | Antiproliferative activity against human umbilical vein endothelial cells (HUVECs) using MTT assay, IC50 = 0.004 μM. | 11591505 | ||
| LoVo cells | Antiproliferative activity assay | Antiproliferative activity against human colon cancer cell line (LoVo cells) using MTT assay, IC50 = 0.001 μM. | 11591505 | ||
| Sf9 insect cells | Function assay | Inhibition of protein kinase C(1:1mixture of PKC alpha & beta) expressed in Sf9 insect cells, IC50 = 0.02 μM. | 9873605 | ||
| endothelial cells | Function assay | Effective dose against plasminogen activator activity stimulated by phorbol ester in endothelial cells, ED50 = 7.5 μM. | 8709095 | ||
| human colon carcinoma cell line HCT116 | Function assay | Concentration required for growth inhibition of human colon carcinoma cell line HCT116, IC50=6 nM. | 15537345 | ||
| MCF7 cells | Function assay | Inhibition of PKA in human MCF7 cells by array-based fluorescence assay, Ki = 0.005 μM. | 18656369 | ||
| MCF7 cells | Function assay | Inhibition of PKA in human MCF7 cells by array-based fluorescence assay, IC50 = 2 μM. | 18656369 | ||
| Sf9 cells | Function assay | Inhibition of human Syk expressed in Sf9 cells, IC50 = 0.003 μM. | 18823784 | ||
| Sf9 cells | Function assay | Inhibition of human ZAP70 expressed in Sf9 cells, IC50 = 0.053 μM. | 18823784 | ||
| insect cells | Function assay | Inhibition of human recombinant Pim1 expressed in insect cells by HTRF, IC50 = 0.01 μM. | 19179076 | ||
| insect Sf21 cells | Function assay | Inhibition of JAK3 expressed in insect Sf21 cells assessed as inhibition of biotinylated substrate phosphorylation, IC50 = 0.006 μM. | 19427203 | ||
| Jurkat cells | Antiproliferative activity assay | Antiproliferative activity against JAK3 expressing IL2-stimulated human Jurkat cells, IC50 = 0.071 μM. | 19427203 | ||
| Jurkat cells | Antiproliferative activity assay | Antiproliferative activity against JAK3 expressing human resting Jurkat cells, IC50 = 0.311 μM. | 19427203 | ||
| HT-29 cells | Function assay | Inhibition of mitochondrial membrane potential in human HT-29 cells using JC1 dye staining by fluorescence plate reader assay, IC50 = 0.0025 μM. | 21513293 | ||
| HCT116 cells | Function assay | Inhibition of TNIK-mediated TCF4/beta-casein transcription in human HCT116 cells by beta-lactamase reporter gene assay, IC50 = 0.039 μM. | 23232060 | ||
| V79 MZ cells | Function assay | Inhibition of human aldosterone synthase expressed in V79 MZ cells assessed as inhibition of aldosterone synthesis, IC50 = 0.011 μM. | 24422519 | ||
| HepG2 cells | Cytotoxicity assay | Cytotoxicity against human HepG2 cells, EC50 = 2 μM. | 25316317 | ||
| Raji cells | Cytotoxicity assay | Cytotoxicity against human Raji cells, EC50 = 2 μM. | 25316317 | ||
| BJ cells | Cytotoxicity assay | Cytotoxicity against human BJ cells, EC50 = 2 μM. | 25316317 | ||
| HEK293 cells | Cytotoxicity assay | Cytotoxicity against HEK293 cells, EC50 = 2 μM. | 25316317 | ||
| PC3 cells | Cytotoxicity assay | Cytotoxicity against human PC3 cells by sulforhodamine B assay, IC50 = 0.017 μM. | 29389122 | ||
| HCT116 cells | Cytotoxicity assay | Cytotoxicity against human HCT116 cells by sulforhodamine B assay, IC50 = 0.055 μM. | 29389122 | ||
| Sf9 cells | Function assay | Inhibition of N-terminal FLAG-tagged human full-length CDK12 (1 to 1490 residues)/N-terminal His-tagged CycK (1 to 580 residues) expressed in Sf9 cells assessed as reduction in ATP-dependent ULight-4E-BP1 (Thr37/Thr46) substrate peptide phosphorylation pr, IC50 = 0.053 μM. | 30067358 | ||
| Sf9 cells | Function assay | Inhibition of N-terminal FLAG-tagged human full-length CDK12 (1 to 1490 residues)/N-terminal His-tagged CycK (1 to 580 residues) expressed in Sf9 cells assessed as reduction in ATP-dependent ULight-4E-BP1 (Thr37/Thr46) substrate peptide phosphorylation pr, IC50 = 0.053 μM. | 30067358 | ||
| Sf9 cells | Function assay | Inhibition of N-terminal FLAG-tagged human full-length CDK13 (1 to 1512 residues)/N-terminal His-tagged CycK (1 to 580 residues) expressed in Sf9 cells assessed as reduction in ATP-dependent ULight-4E-BP1 (Thr37/Thr46) substrate peptide phosphorylation pr, IC50 = 0.057 μM. | 30067358 | ||
| Sf9 cells | Function assay | Inhibition of N-terminal FLAG-tagged human full-length CDK13 (1 to 1512 residues)/N-terminal His-tagged CycK (1 to 580 residues) expressed in Sf9 cells assessed as reduction in ATP-dependent ULight-4E-BP1 (Thr37/Thr46) substrate peptide phosphorylation pr, IC50 = 0.057 μM. | 30067358 | ||
| 他の多くの細胞株試験データをご覧になる場合はこちらをクリックして下さい | |||||
生物活性
| 製品説明 | スタウロスポリン (Staurosporine (AM-2282、Antiviotic AM-2282、STS)) は、PKCα、PKCγ、および PKCη に対する強力な PKC 阻害剤であり、IC50 は 2 nM、5 nM、および 4 nM です。 無細胞アッセイでは、PKCδ (20 nM)、PKCε (73 nM) に対して効力が低く、PKCζ (1086 nM) に対してほとんど活性がありません。 また、PKA、PKG、S6K、CaMKII などの他のキナーゼに対しても阻害活性を示します。臨床フェーズ3。 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Targets |
|
| In Vitro | ||||
| In vitro | Staurosporine (STS), a microbial alkaloid, significantly inhibits protein kinase C from rat brain with IC50 of 2.7 nM. It also displays strong inhibitory effect against HeLa S3 cells with IC50 of 4 nM. This compound inhibits a variety of other protein kinases, including PKA, PKG, phosphorylase kinase, S6 kinase, Myosin light chain kinase (MLCK), CAM PKII, cdc2, v-Src, Lyn, c-Fgr, and Syk with IC50 of 15 nM, 18 nM, 3 nM, 5 nM, 21 nM, 20 nM, 9 nM, 6 nM, 20 nM, 2 nM, and 16 nM, respectively. At 1 μM, it induces >90% apoptosis in PC12 cells. Consistently, this treatment induces a rapid and prolonged elevation of intracellular free calcium levels [Ca2+]i, accumulation of mitochondrial reactive oxygen species (ROS), and subsequent mitochondrial dysfunction. The apoptosis of MCF7 cells induced by STS can be enhanced by the expression of functional caspase-3 via caspase-8 activation and Bid cleavage. Treatment at 1 μM only partially inhibits IL-3-stimulated Bcl2 phosphorylation but completely blocks PKC-mediated Bcl2 phosphorylation. It induces apoptosis of human foreskin fibroblasts AG-1518, depending on the lysosomal cathepsins D mediated cytochrome c release and caspase activation. In addition to activating the classical mitochondrial apoptosis pathway, STS triggers a novel intrinsic apoptosis pathway, relying on the activation of caspase-9 in the absence of Apaf-1. | |||
|---|---|---|---|---|
| Kinase Assay | Enzyme assay and binding assay | |||
| Protein kinase C is assayed in a reaction mixture (0.25 mL) containing 5 μmol of Tris/HCl, pH 7.5, 2.5 μmol of magnesium acetate, 50 μg of histone II S, 20 μg of phosphatidylserine, 0.88 μg of diolein, 125 nmol of CaCl2, 1.25 nmol of [γ-32]ATP (5-10 × 104 cpm/nmol) and 5 μg of partially purified enzyme. The binding of [3H]PDBu to protein kinase C is determined: Reaction mixture (200 μL) contained 4 μmol of Tris/malate, pH 6.8, 20 μmol of KCl, 30 nmol of CaCl2, 20 μg of phosphatidylserine, 5 μg of partially purified protein kinase C, 0.5% (final concentration) of DMSO, 10 pmol of [3H]PDBu (1-3 × 104 cpm/pmol) and 10 μL of various amounts of Staurosporine (STS). | ||||
| 細胞実験 | 細胞株 | PC12 | ||
| 濃度 | Dissolved in DMSO, final concentration 1 μM | |||
| 反応時間 | ~32 hours | |||
| 実験の流れ | Cells are exposed to Staurosporine (STS) for ~32 hours, after which they are fixed in 4% paraformaldehyde and stained with the DNA-binding dye Hoechst 33342. Under epifluorescence illumination, the percentage of apoptotic cells (cells with condensed and fragmented DNA) is determined. |
|||
| 実験結果図 | Methods | Biomarkers | 結果図 | PMID |
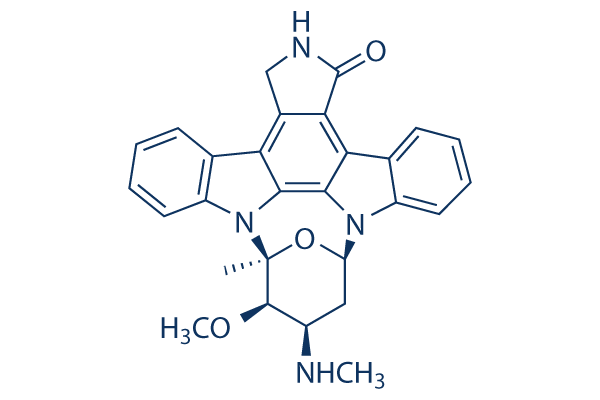
| Western blot | FAK / RIP p-Akt / Akt / PARP /Cleaved PARP Ambra1 |
|
15121855 | |
| Growth inhibition assay | Cell viability |

|
25215174 | |
| Immunofluorescence | Phalloidin / Type II collagen Tubulin / Actin FAK 4.47 cleaved-caspase 3 Annexin Pyk2 |
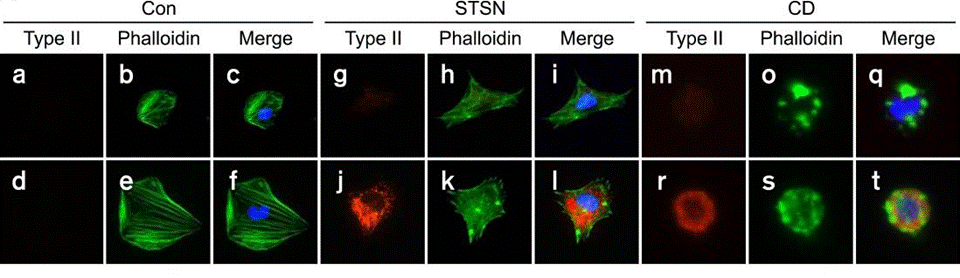
|
22684244 | |
| In Vivo | ||
| In Vivo | In the gerbil and rat ischemia models, pretreatment with Staurosporine (STS) (0.1–10 ng) before ischemia prevents neuronal damage in a dose-dependent manner, suggesting the involvement of PKC in CA1 pyramidal cell death after ischemia. | |
|---|---|---|
| 動物実験 | 動物モデル | Male Mongolian gerbils or male Wistar rats subjected to transient ischemia |
| 投与量 | ~10 ng | |
| 投与経路 | Stereotaxically administered into the bilateral CAl subfield of the hippocampus | |
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05209347 | Withdrawn | Ankle Fractures|Post-traumatic Osteoarthritis|Osteoarthritis Ankle |
University of Iowa |
December 2024 | Not Applicable |
| NCT05956990 | Not yet recruiting | Colorectal Cancer Survivor|Rehabilitation|Quality of Life |
Qu Shen|The First Affiliated Hospital of Xiamen University|Xiamen University |
July 1 2024 | Not Applicable |
| NCT05957289 | Not yet recruiting | Rehabilitation|Colorectal Cancer Survivor|Quality of Life|Pilot Study |
Qu Shen|The First Affiliated Hospital of Xiamen University|Xiamen University |
June 1 2024 | Not Applicable |
| NCT06245135 | Not yet recruiting | Mobility Limitation |
University of Toronto|University Health Network Toronto|March of Dimes Canada|Heart and Stroke Foundation of Canada|University of Alberta|University of Manitoba|Bruyere Research Institute |
June 2024 | Not Applicable |
| NCT06374108 | Not yet recruiting | Multiple Sclerosis |
University of Aarhus|Aarhus University Hospital|University of Copenhagen |
May 1 2024 | Not Applicable |
|
化学情報
| 分子量 | 466.53 | 化学式 | C28H26N4O3 |
| CAS No. | 62996-74-1 | SDF | Download Staurosporine (STS) SDFをダウンロードする |
| Smiles | CC12C(C(CC(O1)N3C4=CC=CC=C4C5=C6C(=C7C8=CC=CC=C8N2C7=C53)CNC6=O)NC)OC | ||
| 保管 | |||
|
In vitro |
DMSO : 46 mg/mL ( (98.6 mM); 吸湿したDMSOは溶解度を減少させます。新しいDMSOをご使用ください。) Water : Insoluble Ethanol : Insoluble |
モル濃度計算器 |
|
in vivo Add solvents to the product individually and in order. |
投与溶液組成計算機 | |||||
実験計算
投与溶液組成計算機(クリア溶液)
ステップ1:実験データを入力してください。(実験操作によるロスを考慮し、動物数を1匹分多くして計算・調製することを推奨します)
mg/kg
g
μL
匹
ステップ2:投与溶媒の組成を入力してください。(ロット毎に適した溶解組成が異なる場合があります。詳細については弊社までお問い合わせください)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
計算結果:
投与溶媒濃度: mg/ml;
DMSOストック溶液調製方法: mg 試薬を μL DMSOに溶解する(濃度 mg/mL, 注:濃度が当該ロットのDMSO溶解度を超える場合はご連絡ください。 )
投与溶媒調製方法:Take μL DMSOストック溶液に μL PEG300,を加え、完全溶解後μL Tween 80,を加えて完全溶解させた後 μL ddH2O,を加え完全に溶解させます。
投与溶媒調製方法:μL DMSOストック溶液に μL Corn oil,を加え、完全溶解。
注意:1.ストック溶液に沈殿、混濁などがないことをご確認ください;
2.順番通りに溶剤を加えてください。次のステップに進む前に溶液に沈殿、混濁などがないことを確認してから加えてください。ボルテックス、ソニケーション、水浴加熱など物理的な方法で溶解を早めることは可能です。
技術サポート
ストックの作り方、阻害剤の保管方法、細胞実験や動物実験の際に注意すべき点など、製品を取扱う時に問い合わせが多かった質問に対しては取扱説明書でお答えしています。
他に質問がある場合は、お気軽にお問い合わせください。
* 必須
